Glossary
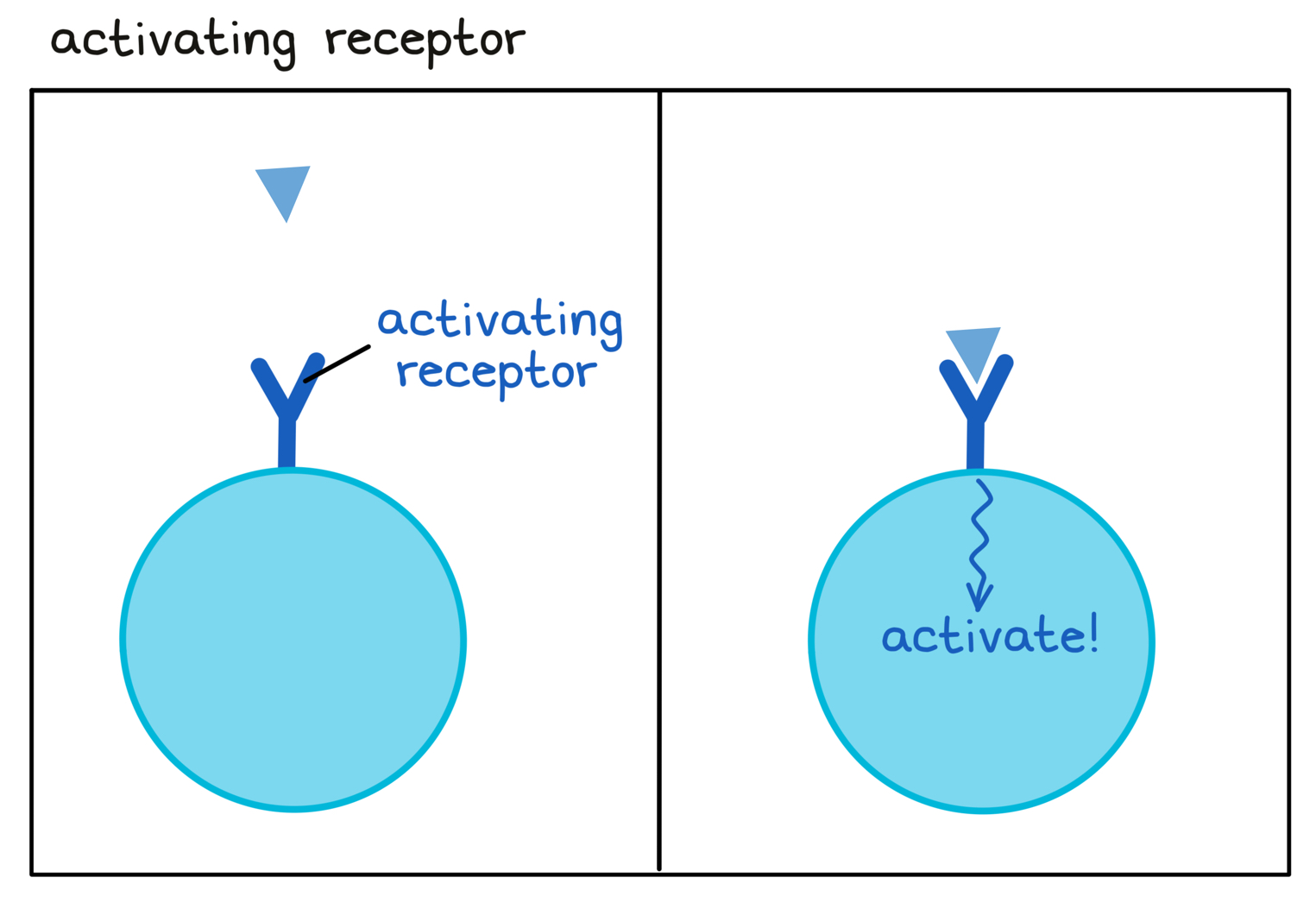
- Activating receptor
A receptor on an immune cell that enhances or speeds up the activity of the immune cell.
- Adaptive immune system
One of the two arms of the immune system. The adaptive immune system includes cells (mainly T cells and B cells) and antibodies that are highly specific to a very wide range of different targets. Adaptive immune responses are long-lasting. This “memory” allows an individual to quickly respond and fight off the threat when it is encountered again.
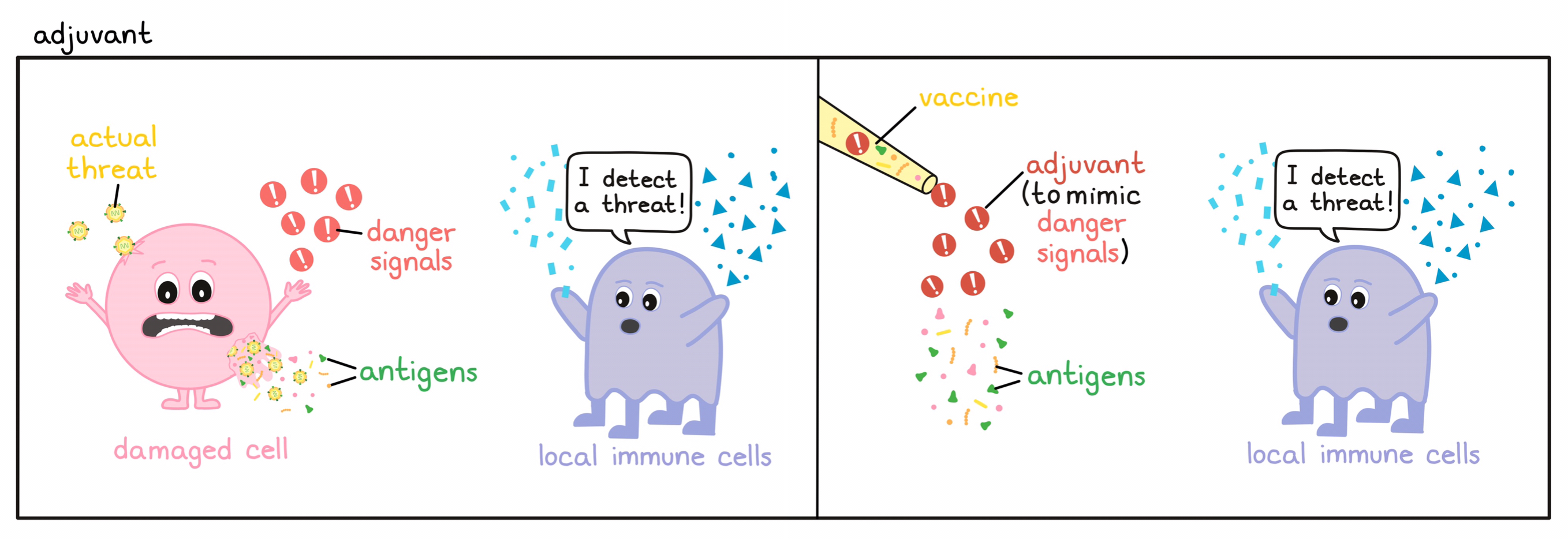
- Adjuvant
A substance that can improve the effect of a vaccine and boost the immune response against the antigens in the vaccine. Adjuvants often mimic the natural “danger signals” that are present during a viral or bacterial infection and can alert the immune system, usually by stimulating antigen-presenting cells to more strongly activate T cells or B cells.
- Adjuvant therapy
Additional therapy that is given after the main therapy has completely eliminated all detectable cancer. For example, if a patient undergoes a surgery to completely remove a tumor, they may receive some other therapy afterwards to help destroy any remaining cancer cells that might have been missed by the main therapy. Many different types of therapy can be used as an adjuvant therapy, including immunotherapy.
- Adoptive cell therapy
A type of immunotherapy in which immune cells (T cells or NK cells)are removed from the patient (or donor), manipulated in the laboratory to increase their ability to directly eliminate cancer cells, and returned to the patient. Sometimes, the immune cells are genetically modified in the lab to change the way the immune cells target cancer cells. Types of adoptive cell therapy include:
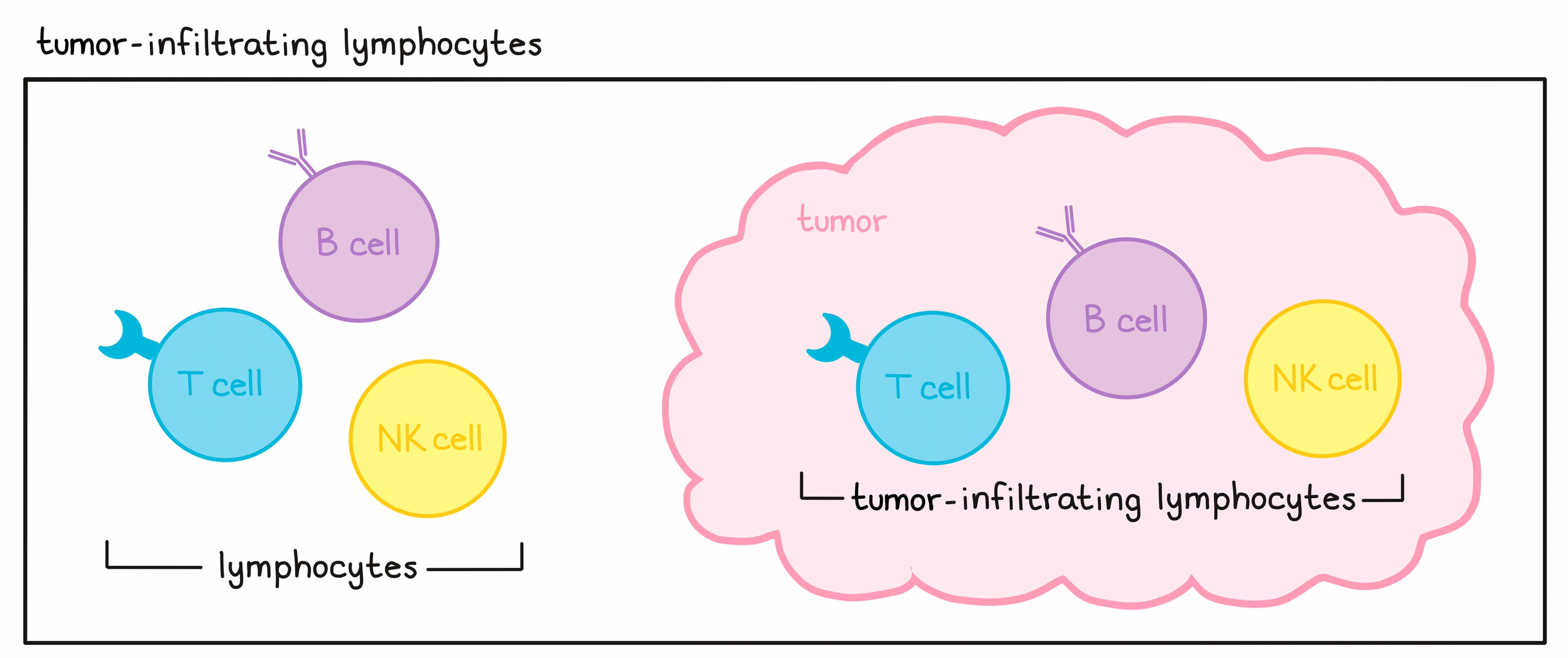
- Tumor-Infiltrating Lymphocyte therapy: makes use of T cells found within tumors, which may naturally have T cell receptors that recognize targets on cancer cells. View Tumor-Infiltrating Lymphocyte.
- T cell receptor-engineered T cell therapy: makes use of genetic engineering of a patient’s T cells to add alternative T cell receptors that recognize targets on cancer cells.
- CAR T cell therapy: makes use of genetic engineering to endow a patient’s T cells with artificial receptors known as “CARs” which can recognize targets on cancer cells that are invisible to T cell receptors. View CART T cell therapy.
- CAR NK cell therapy: makes use of genetic engineering to endow a patient’s NK cells with artificial receptors known as “CARs” which can recognize targets on cancer cells that are invisible to unmodified NK cells.
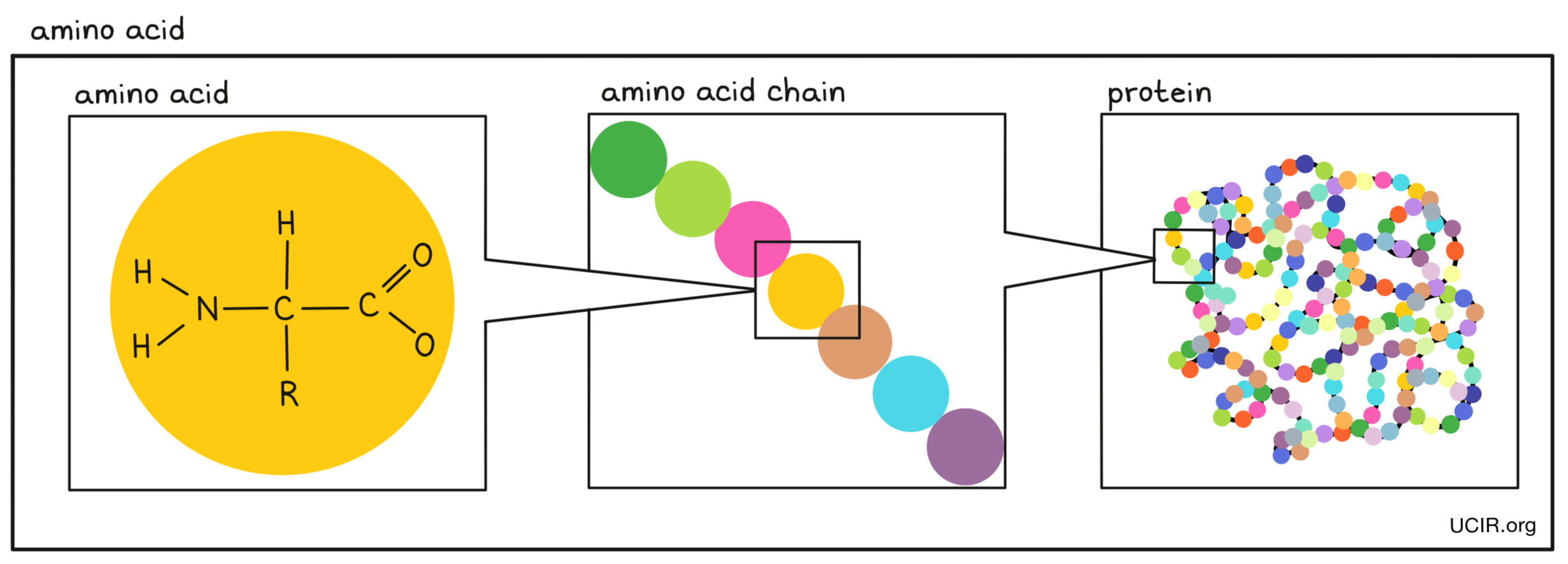
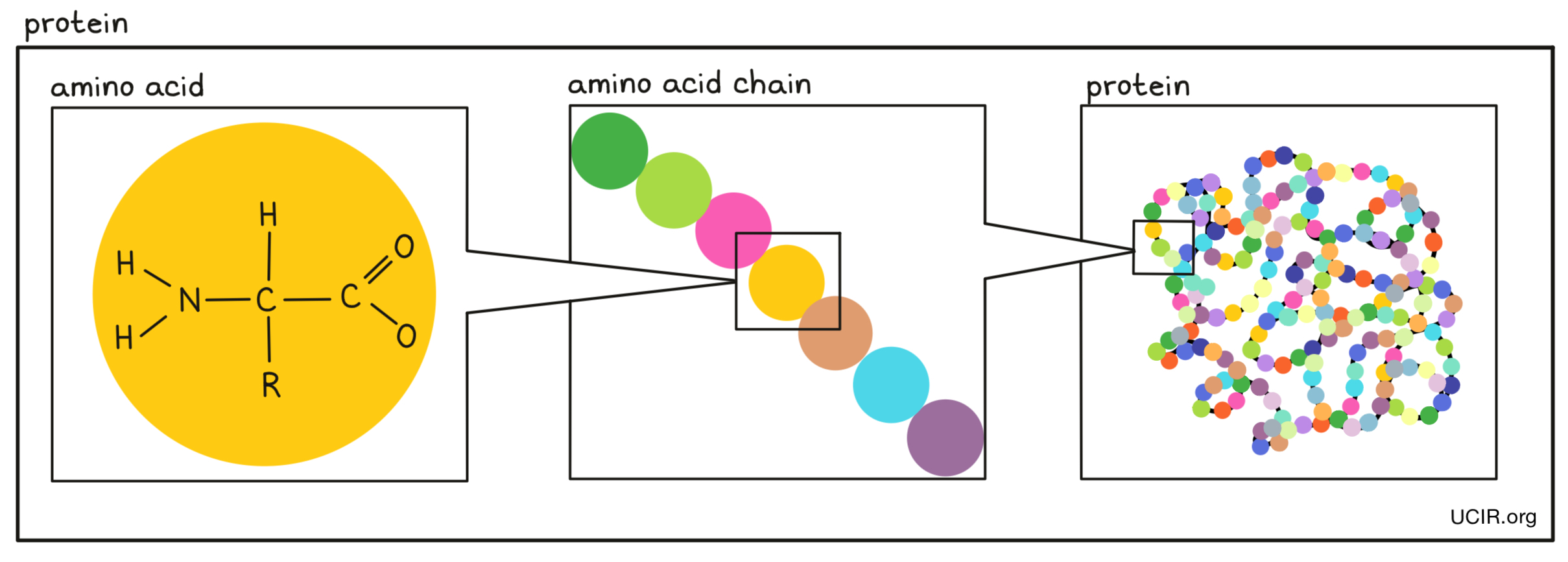
- Amino acids
Small building blocks that are joined together in a string to make proteins. There are 20 different amino acids. Each amino acid has a core that is the same in all amino acids and is used to link amino acids together, and a side chain that is different between amino acids and gives each amino acid a unique chemical property.
- Anaplastic lymphoma receptor tyrosine kinase (ALK)
The ALK gene provides the instructions to generate the ALK protein, which is present on the cell surface and involved in sending signals that direct cells to multiply. The ALK protein is usually only active during prenatal development. Cancer-causing mutations in the ALK gene, including cases in which the ALK gene fuses (joins) with parts of another gene, may abnormally turn on the ALK protein and keep it on, causing cells to start to multiply uncontrollably. ALK mutations have been associated with several types of cancer, including some non-small cell lung cancers, some neuroblastomas, and some lymphomas (in particular, anaplastic large cell lymphomas). Therapies called ALK inhibitors, such as crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib, can help slow down tumor growth.
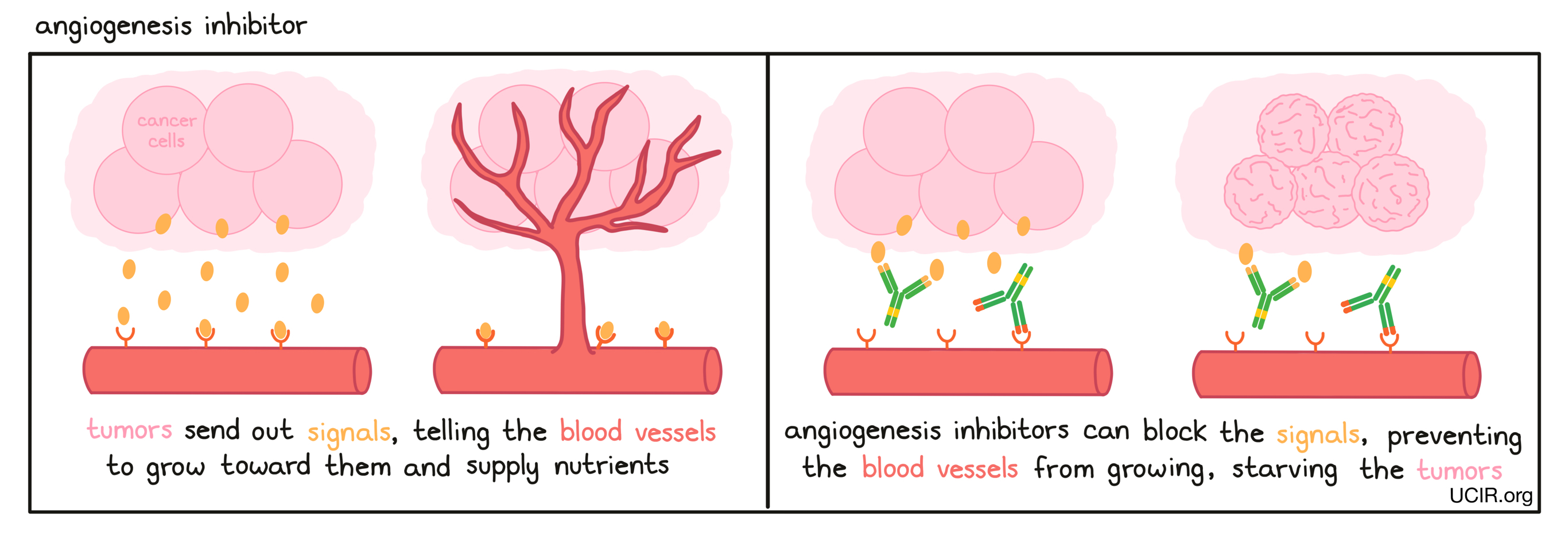
- Angiogenesis inhibitor
A drug that prevents the growth of new blood vessels, which are needed for tumors to grow. Examples of such drugs include Inlyta (axitinib), Sutent (sunitinib), and Avastin (bevacizumab).
- Antibody
A protein molecule produced by B cells that binds to a particular threatening target (antigen). In cancer, antibodies can stop or slow down tumor growth, stop cancer cells from spreading to other parts of the body, mark cancer cells for elimination by the immune system, or amplify an immune response. Each antibody recognizes only a single type of target and each B cell produces only a single type of antibody.
Antibodies have a characteristic “Y” shape, and the ends of each of the two upper arms of the “Y” are parts of the antibody that specifically recognize its target, which is often found on the surface of a cell, such as a cancer cell. The stem of the “Y” is recognized by immune cells or other parts of the immune system, and it forms a bridge between the target and the immune system.
Antibodies can be manufactured in the lab and are commonly used in antibody therapy to target cells which produce a particular molecule (antigen) that distinguishes them from normal, healthy cells.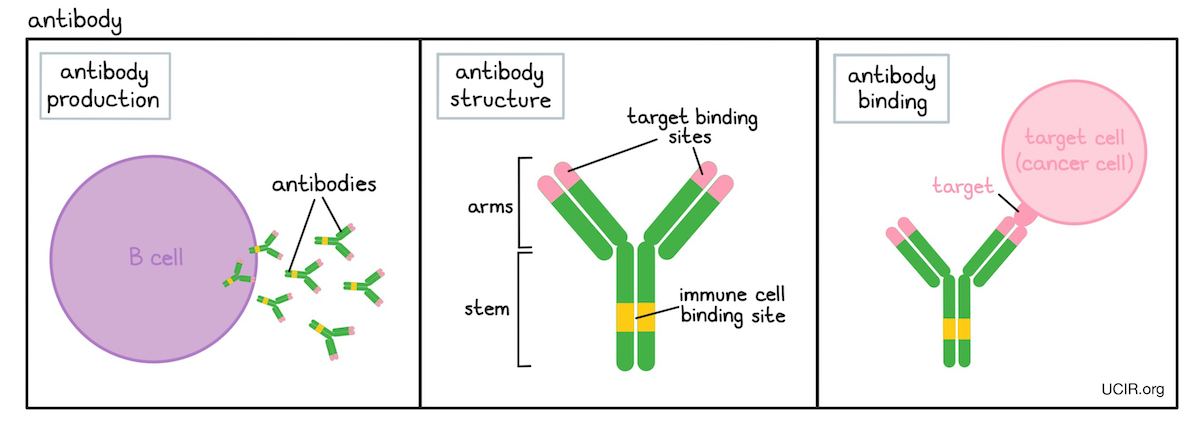
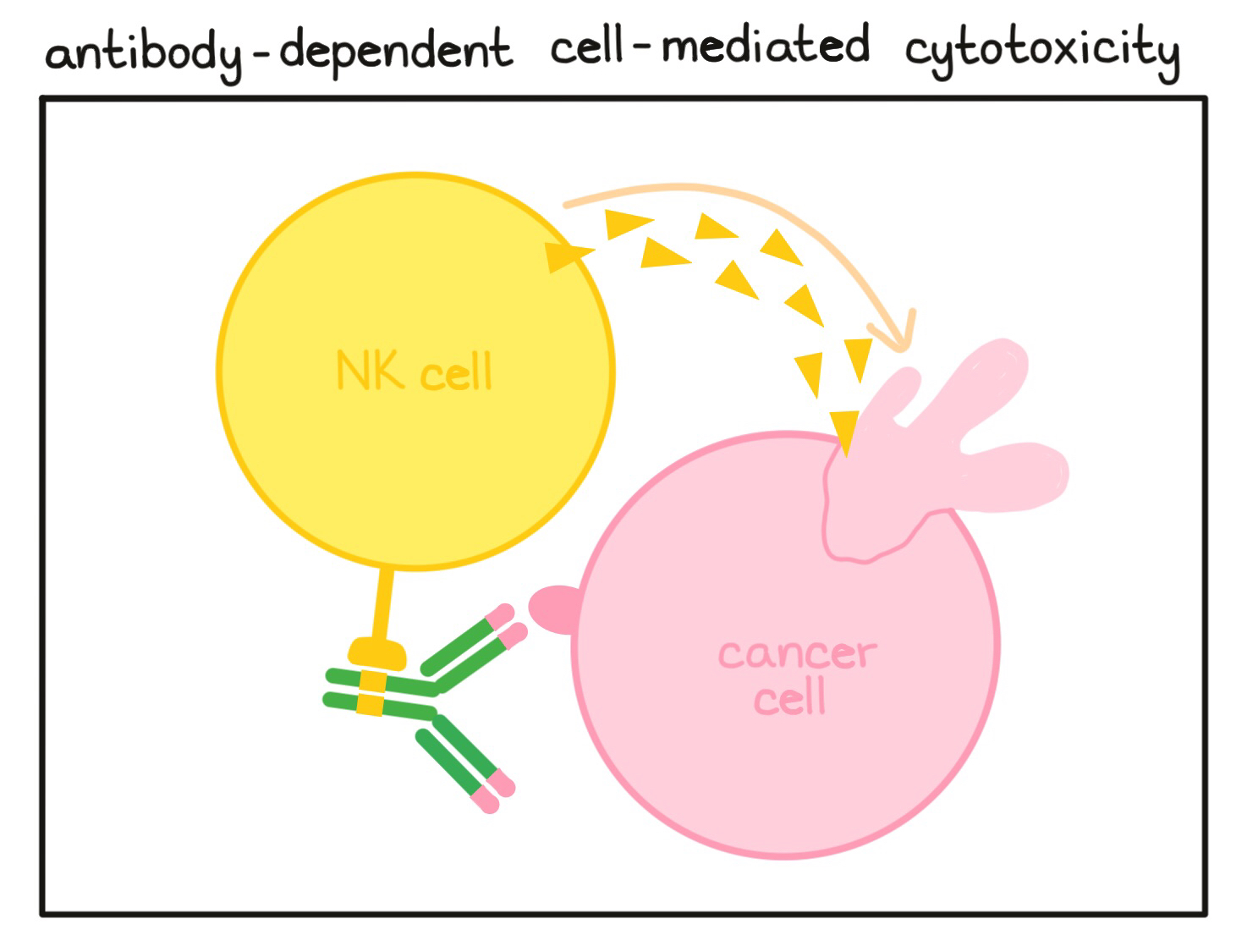
- Antibody-dependent cell-mediated cytotoxicity (ADCC)
A cell-killing process in which antibodies that bind to targets on a cell attract NK cells (or other immune cells) which can then destroy the antibody-coated cell.
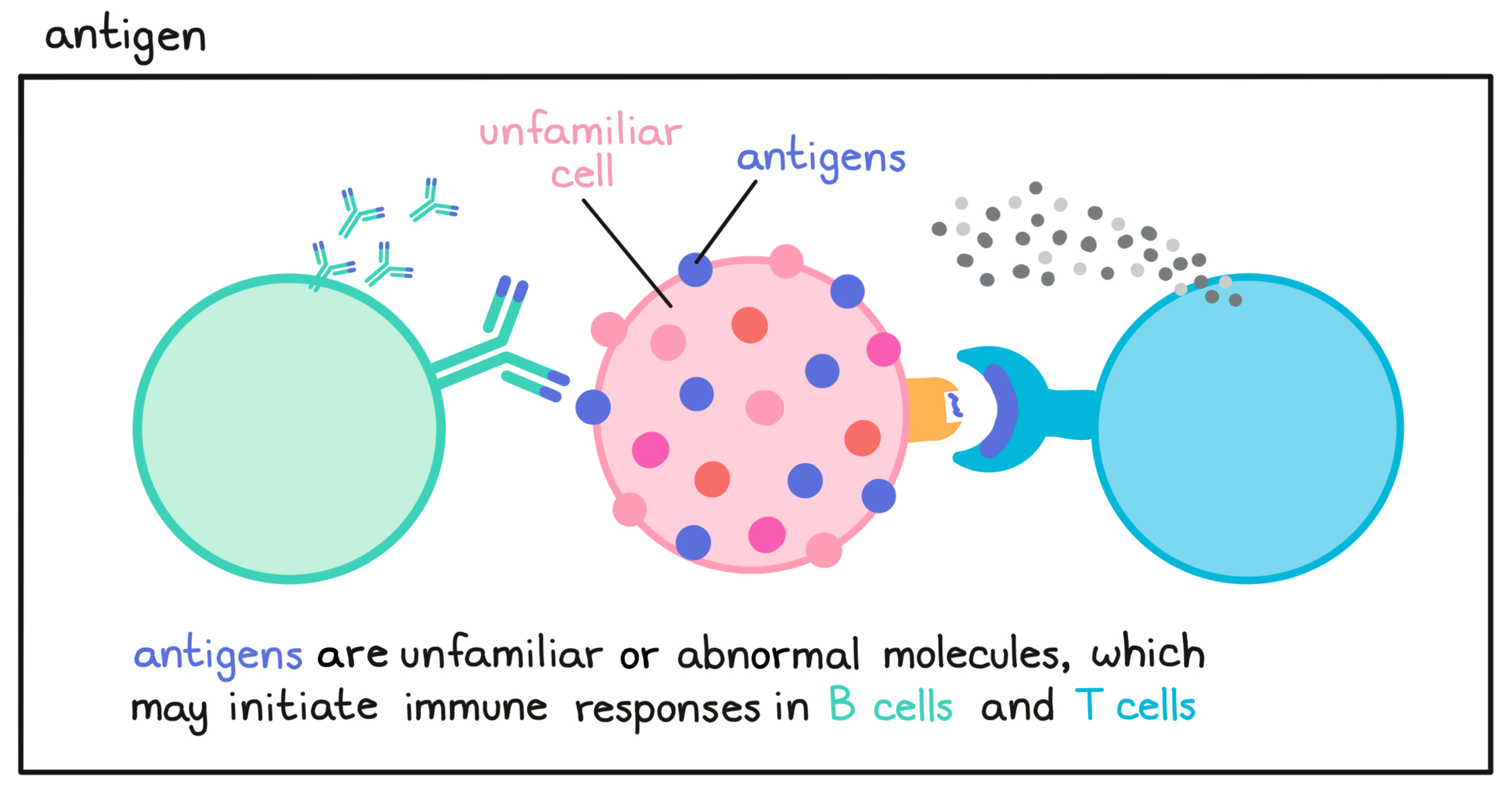
- Antigen
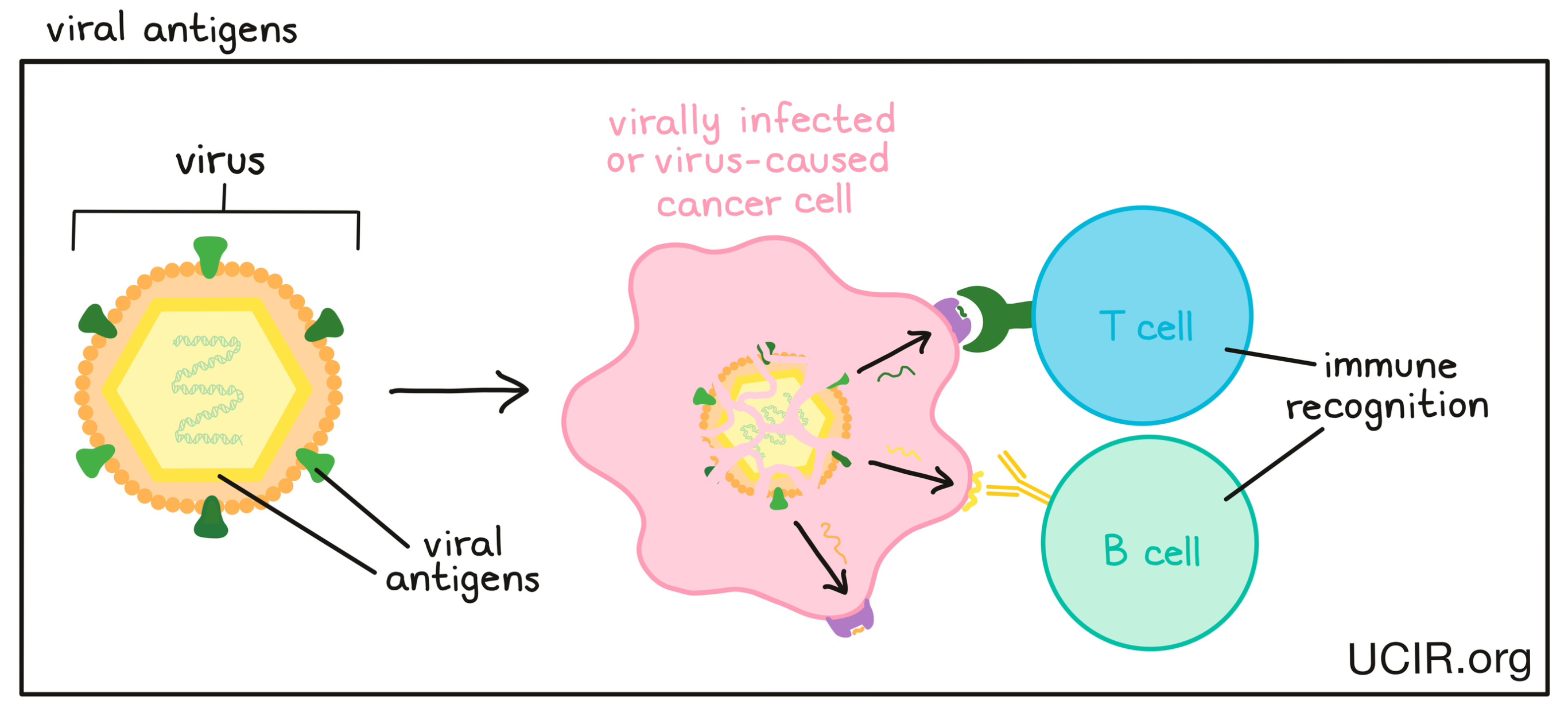
A molecule that is abnormal or unfamiliar to the body (e.g., a cancer antigen, a neoantigen or viral antigen) and stimulates an immune response involving T cells or B cell.
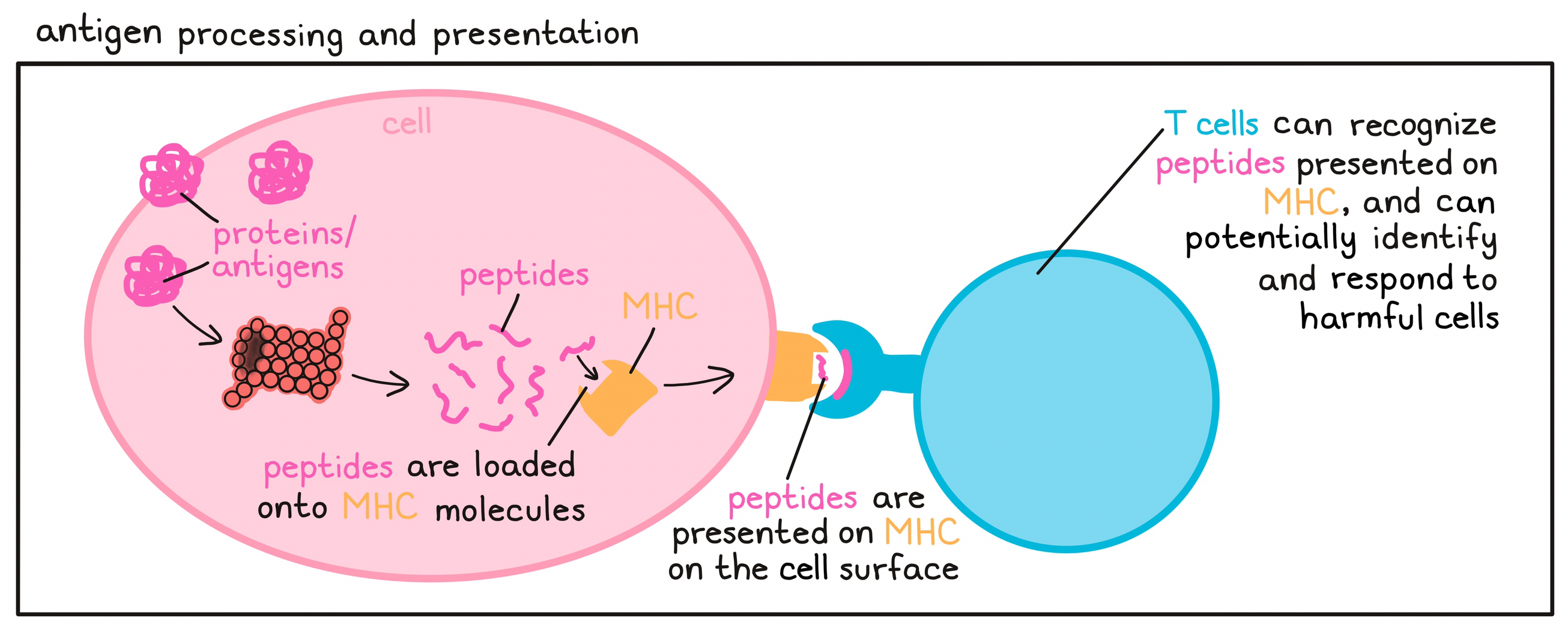
- Antigen processing and presentation
The mechanism by which protein molecules (including antigens) within a cell are broken down into smaller fragments (peptides) that can be recognized by T cells – the key active warriors in the immune system’s fight against cancer. The peptides are loaded onto MHC molecules and moved to the cell surface. T cells (with their T cell receptor) can only recognize protein fragments that are presented in this way, and can potentially identify and respond to harmful cells
- Antigen-presenting cell (APC)
A type of immune cell that is able to present evidence of threats (abnormal molecules from unhealthy or infected cells) to T cells – the key active warriors in the immune system’s fight against cancer. APCs are able to ingest (“eat”) dying or unhealthy cells, invading viruses, or bacteria, and to digest the protein molecules in what they ate into smaller fragments (peptides). The peptides then are loaded onto MHC molecules and moved to the cell surface of the APC where they can be seen by T cells. If a particular T cell recognizes the peptide as evidence of a threat (antigen), it will become activated and stimulated to multiply into a T cell army that ultimately attacks the threatening cells. There are many types of antigen-presenting cells including dendritic cells, macrophages, and monocytes (a type of macrophage).
- Autoimmune
A term used to describe an immune response against normal, healthy cells. The immune system is programmed to attack and destroy foreign invaders, cells that have been damaged, or cells like cancer that are threatening to the body. An autoimmune response occurs when the immune system mistakes normal, healthy cells for threats and attacks them. Autoimmune responses can range from mild to life-threatening.
- Autophagy
A process that occurs in cells that are stressed or starved for nutrients by which the cell begins to digest parts of itself to supply these nutrients or to prepare the cell for death.
A
- B Cell
A type of immune cell that can recognize abnormal molecules (antigens) on unhealthy cells, viruses, bacteria, or other threats, and can produce antibodies, which bind to the antigen. In cancer, antibodies can stop or slow down tumor growth, stop cancer cells from spreading to other parts of the body, mark cancer cells for elimination by the immune system, or amplify an immune response. Each antibody can recognize only one particular antigen and each B cell produces only a single type of antibody. Antibodies can be used for antibody therapy.
- BRAF
The BRAF gene provides the instructions to generate the BRAF protein, which is found inside certain cell types and is involved in sending signals that direct cells to multiply. Mutations in the BRAF gene may cause the BRAF protein to be active all the time, so that cells start to multiply uncontrollably. BRAF mutations have been associated with various cancers, including some non-Hodgkin lymphomas, colorectal cancers, melanomas, thyroid cancers, lung cancers, and others. Therapies called BRAF inhibitors, such as dabrafenib, encorafenib, and vemurafenib, can help slow down tumor growth.
- Basophil
A type of immune cell that is typically found in tissues and is involved in responding to certain parasitic infections and allergens. Basophils contributes to inflammatory responses by releasing a molecule called histamine, which leads to leakiness in small blood vessels, allowing other immune cells to enter the site of inflammation. Basophils are a type of granulocytes.
- Bioinformatic analysis
An analysis (usually involving advanced algorithms) of large amounts of biological information from a patient or multiple patients, particularly involving their DNA, RNA, and protein sequences. In cancer immunotherapy, this type of analysis can be used to investigate the mutations and alterations within an individual patient’s cancer. The information that is obtained through this type of analysis can help doctors predict which treatments are likely to work best for a patient and, in some cases, may help them to develop personalized immunotherapy treatments.
- Biomarker
A measurable indicator for whether a patient is likely to respond to a particular therapy. Biomarkers are representative of conditions within a patient’s body, and in some cases within the cancer, that may impact how well a particular treatment will work. Strong biomarkers can help doctors determine the best possible treatment plan for an individual patient.
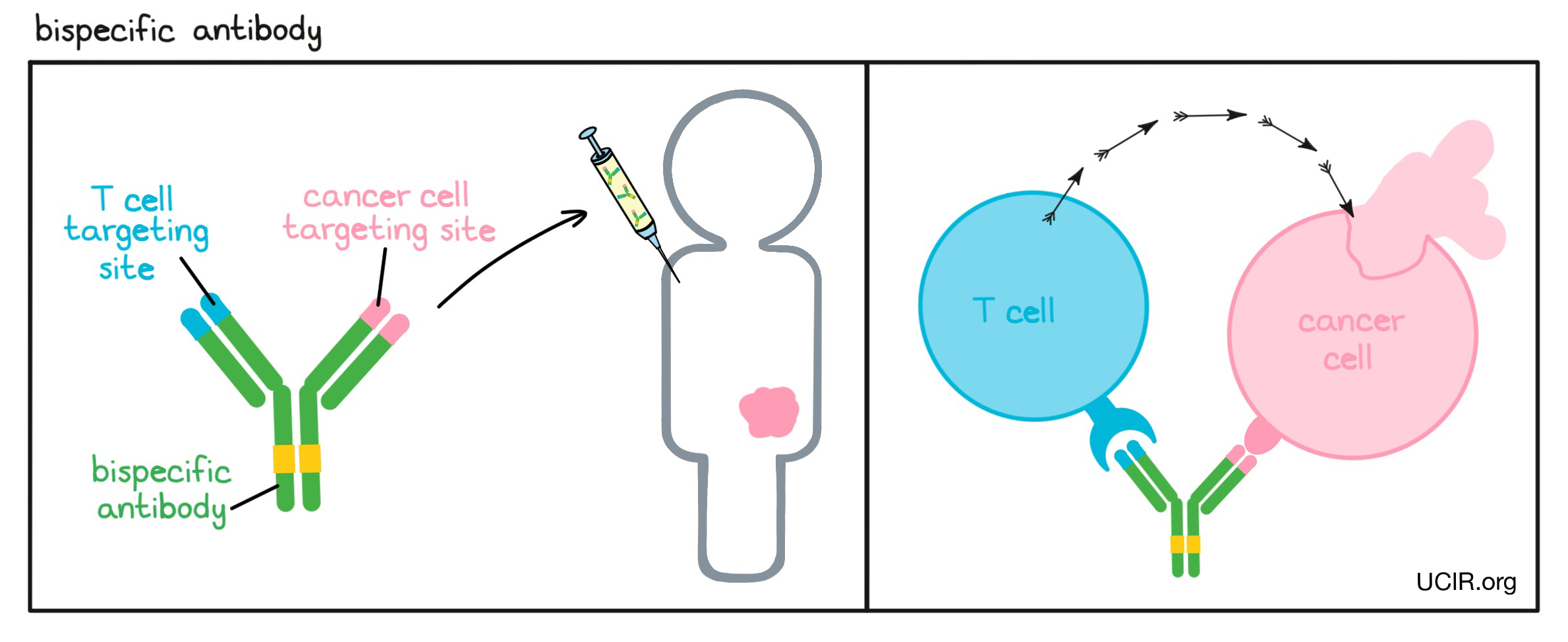
- Bispecific antibody
A type of artificial antibody produced in laboratories that can simultaneously recognize and bind to two different targets at once. Bispecific antibodies are typically used to bring different cell types (like an immune cell and a cancer cell) into close proximity by binding to a target on the cancer cell and a target on an immune cell. Binding of the bispecific antibody activates the immune cell to destroy the cancer cell. T cells are the major immune cells responsible for cancer cell killing and are typically the target of a bispecific antibody.
- Blood-brain barrier
A barrier made of blood vessels and tightly packed cells that helps prevent harmful substances (such as bacteria) in the blood from reaching the brain. Some substances, such as water and oxygen, can cross the blood-brain barrier and enter the brain, however, many anticancer drugs are kept out. The blood-brain barrier can sometimes be partially disrupted by diseases such as cancer.
- Bone marrow
The soft, spongy tissue found in the cavities of most bones, where blood and immune cells are made. Bone marrow contains hematopoietic stem cells that can become red blood cells, immune cells, or platelets, as well as stem cells that can become cartilage, fat, or bone cells. Blood and immune cells are shipped out to the rest of the body through the many blood vessels that run through the bone marrow.
B
- CAR T Cell
A T cell that has been genetically modified to display an artificial receptor called chimeric antigen receptor (CAR) on its surface. T cells are the major cells of the immune system responsible for seeking out and destroying unhealthy cells. However, not every T cell can recognize cancer cells. Modification of the patient’s own T cells with a CAR creates a “living drug” that can be used to eliminate cancer cells. CAR T cells are a major type of T cell therapy.
- CD4+ T cells
One of the two major subsets of T cells. CD4+ and CD8+ T cells account for more than 95% of the T cells in a typical individual. There are multiple types of CD4+ T cells including “helper” T cells, which enhance the function of antigen-presenting cells, and regulatory T cells, which are involved in dampening immune reactions.
- CD8+ T cells
One of the two major subsets of T cells. CD8+ and CD4+ T cells account for more than 95% of the T cells in a typical individual. Using their T cell receptor, CD8+ T cells can recognize abnormal or threatening cells, and are specialized in killing such cells.
- CTLA-4
A protein molecule on the surface of newly activated T cells that can bind to molecules (CD80 or CD86) on the surface of antigen-presenting cells. Binding between CTLA-4 and CD80 or CD86 prevents further activation of T cells and limits the ability of the T cells to attack the tumor. Using antibodies to block binding between CTLA-4 and CD80 or CD86 is a common strategy in immunotherapy (checkpoint blockade) to allow further T cell activation and enhance cancer cell killing.
CTLA-4 is also highly present on the surface of regulatory T cells (Tregs), a type of T cell that suppresses the activity of other T cells. Binding of antibodies to CTLA-4 on Tregs can lead to the elimination of Tregs, which can also result in stronger anti-cancer activity.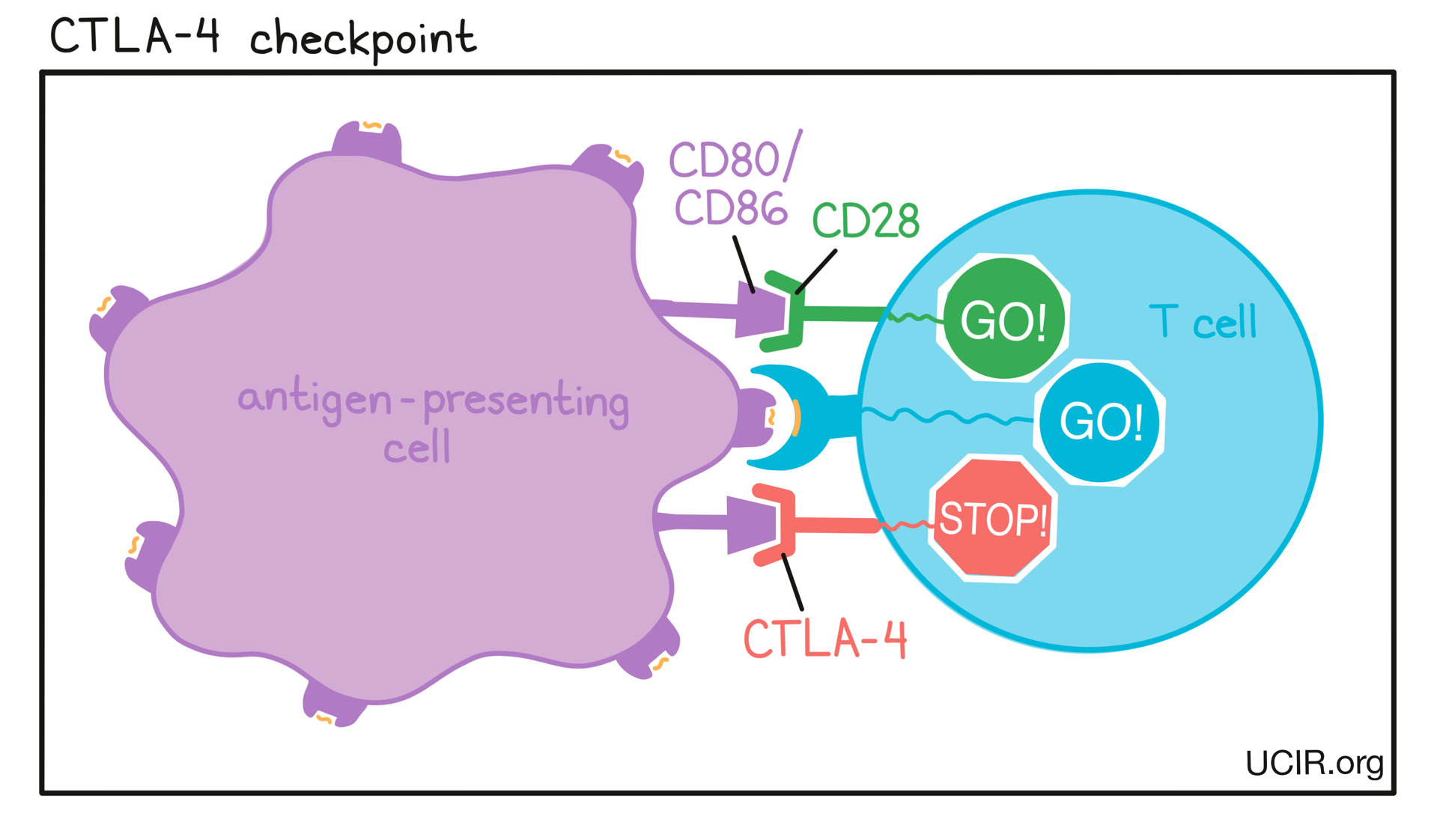
- Cancer
A term for diseases in which abnormal, mutated cells multiply out of control, with the potential to invade nearby tissues and spread to other parts of the body through the blood and lymphatic system (metastasize), also called malignancy. The main types of cancer are:
- Carcinomas originate in the skin or in tissues that line or cover internal organs.
- Sarcomas originate in the bone, cartilage, fat, muscle, blood vessels, or other connective or supportive tissue.
- Leukemias originate in blood-forming tissues, such as bone marrow, causing the production of large numbers of abnormal blood cells that enter the blood.
- Lymphomas and multiple myeloma originate in the cells of the immune system.
- Central nervous system cancers originate in the tissues of the brain and spinal cord.
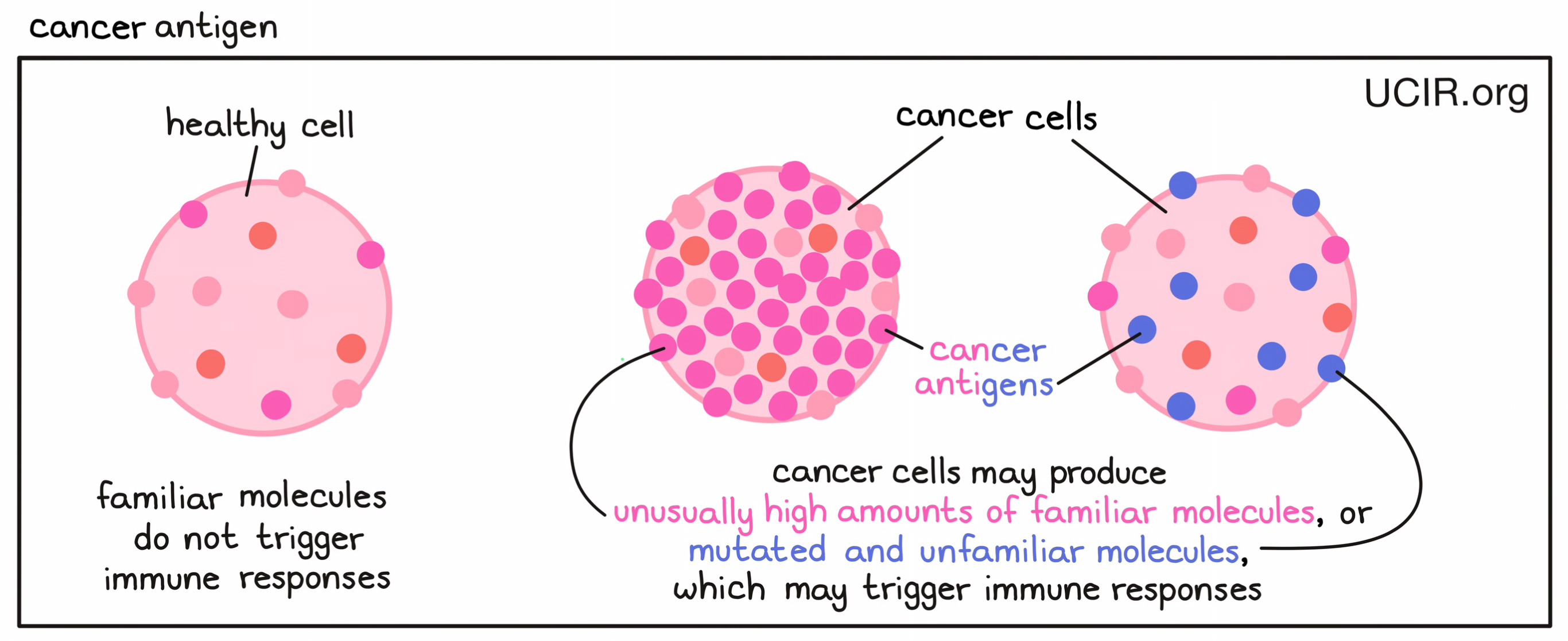
- Cancer antigen
A molecule found in cancer cells that the immune system may recognize as being abnormal or unfamiliar, and that can stimulate an antitumor immune response involving T cells or B cells. Cancer antigens may include familiar molecules that are produced in unusually high amounts, which may catch the immune system’s attention. They may also include mutated and unfamiliar molecules that the immune system interprets as “foreign” to the body (e.g., a neoantigen or viral antigen), and that would be likely to trigger a stronger immune response.
- Cancer germline antigen/cancer testis antigen
A molecule or part of a molecule that is normally only produced during prenatal development or in germ cells (sperm and egg), but is abnormally produced in cancer cells. Because these antigens are not found in normal adult cells, they can be used to distinguish cancer cells from normal cells and can function as targets for immune cells – T cells and B cells – in cancer vaccines.
- Cancer stages
Categories used to define how advanced a patient’s cancer is at the time of diagnosis. Depending on the type of cancer, different staging systems may be used. The simplest and most commonly used staging system identifies four stages – I, II, III, or IV – with stage I being the least advanced and stage IV being the most advanced. Stage I, II, and III cancers are confined to a single site, although in stage III the cancer may have spread to local lymph nodes. Stage IV indicates that the cancer has become metastatic, meaning it has spread to other parts of the body. Other staging systems provide additional subcategories to further define the details of a patient’s cancer.
- Carcinogen
Any substance or radiation (such as sunlight or X-rays) that causes changes or mutations in cells and potentially, the formation of cancer.
- Carcinoma
A type of cancer that originates in cells that make up the skin or lining of organs. There are several subtypes of carcinoma, which can be identified based on how the carcinoma cells look under the microscope. These subtypes include squamous cell, adenosquamous, large cell, small cell, and anaplastic or undifferentiated carcinoma, as well as adenocarcinoma. Carcinomas can form at different sites in the body, such as lung, breast, prostate, colon/rectum, pancreas, ovaries, liver, kidney, and head/neck.
- Cell growth
This term can have two meanings. Most of the time, “cell growth” refers to increasing the number of cells in a cell population. Cell populations “grow” or “multiply” when individual cells divide into two “daughter cells”, allowing the population to increase in numbers. Occasionally, cell growth refers to an individual cell getting larger in size.
- Checkpoint blockade
A strategy to overcome the natural process of keeping an immune reaction under control. The immune system has multiple checkpoints that serve as safety mechanisms to prevent an immune overreaction that can threaten healthy cells. In the case of cancer however, scientists want to enhance the immune response, and so blocking one or more of these checkpoints can be an effective strategy to strengthen the response against cancer cells. Checkpoint blockade brings some risk of damaging healthy cells.
- Chemokine
A small protein secreted by a cell that attracts and activates immune cells, causing them to migrate toward the source of the chemokine. Chemokines are an important part of the inflammatory response, and help to draw in immune cells (lymphocytes, such as T cells and NK cells; and phagocytes, such as macrophages).
- Chemotherapy
A treatment for cancer that uses chemicals to kill fast-multiplying cells in a patient. Most chemotherapy drugs work by halting the process through which cancer cells multiply, and/or by directly killing cancer cells. In contrast, most immunotherapy drugs work by encouraging or enhancing an immune response against cancer cells. In some cases, the damage inflicted by chemotherapy helps to kickstart the immune system, which may have an immunotherapeutic effect. Chemotherapy can also be used to deplete immune cells prior to the administration of certain immunotherapy drugs, or may be used in combination with immunotherapy drugs.
- Clonal expansion
A process by which T cells or B cells with specificity for a single target (antigen) are stimulated to multiply to produce thousands of T cell or B cell copies with the same specificity.
- Compassionate use
Refers to the treatment of severely ill patients with a drug that is either not yet approved or is approved for a different indication when the patients do not have any other viable treatment options available to them. These patients usually do not meet the criteria for entry into a clinical trial, but could still potentially benefit from the experimental treatment as a last resort.
- Complement system
A set of proteins that act together to attack viruses, bacteria, or other threats, and help to clear damaged or unhealthy cells. Activation of the complement system can occur spontaneously in response to certain threats or can be triggered by antibodies that are bound to the threat. Proteins of the complement system can kill certain threats directly or can flag them for removal by phagocytes – specialized immune cells that can ingest threats which are coated with proteins of the complement system.
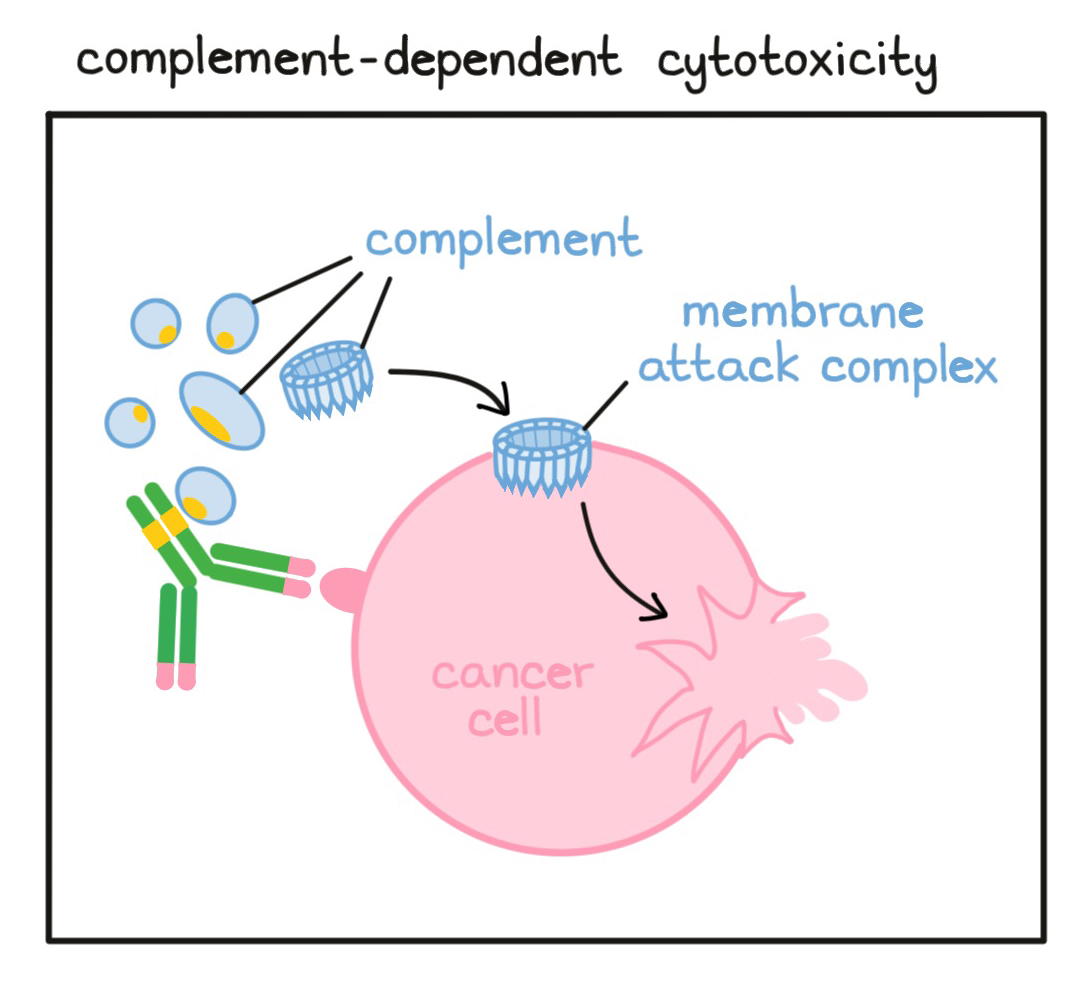
- Complement-dependent cytotoxicity (CDC)
A process by which antibodies (released by B cells) bind to a target cell (usually a cell that doesn’t belong in the body) and attract and activate free-floating molecules called “complement”. These complement molecules can then form a unit called the “membrane attack complex” that punctures the target cell and leads to its death.
- Conditioning
A pre-treatment regimen that may include chemotherapy, monoclonal antibody therapy, and/or radiation prior to a hematopoietic stem cell transplant (HSCT) or cellular therapy. Conditioning is used to kill off cells in the blood and bone marrow in order to eliminate cancer cells, limit transplant rejection, and/or to make room for newly transplanted cells to survive and thrive in the body with less competition for resources. The side effects from this part of the treatment can be severe.
- Consolidation therapy
Additional therapy that is given after the main therapy has completely removed or eliminated all detectable tumor. Consolidation therapy has a meaning very similar to adjuvant therapy, but is often used when discussing blood cancers such as leukemia or lymphoma. Radiation, chemotherapy, stem cell transplants, and immunotherapies like checkpoint blockades can all be used as potential consolidation therapies.
- Costimulatory molecule
A type of molecule on the surface of antigen-presenting cells that can amplify the stimulation of T cells – the key active warriors in the immune system’s fight against cancer. T cells require at least two signals to become fully activated. As a first signal, the antigen-presenting cell has to provide evidence of a threat that can be recognized by the T cell. The second signal is then provided by the costimulatory molecules on the antigen-presenting cells.
- Curative treatment
A treatment or therapy with the potential to completely eliminate the patient's cancer.
- Cytokine
A small protein released by a cell that acts as a signal and affects other cells. Sometimes, a cell secretes cytokines to affect itself. Cytokines made by white blood cells (leukocytes) are often called interleukins (abbreviated IL). Interferons (abbreviated IFN) are another important group of cytokines.
- Cytokine release syndrome (CRS)
A potentially life-threatening condition that requires immediate treatment. CRS is triggered by excessive T cell killing of target cells that results in a widespread release of molecules called cytokines. Cytokines are involved in inflammation and can affect the function of various organs. Signs and symptoms of CRS include high fever, chills, difficulty breathing, fast or irregular heartbeat, severe nausea, vomiting, diarrhea, very low blood pressure, low oxygen level, and dizziness or lightheadedness. CRS is frequently observed with CAR T cell therapies or with bispecific antibodies.
C
- DNA
A type of molecule that carries the genetic information of a cell and determines many biological traits. DNA is found in all living things and in nearly all cells in the body. It serves as the instruction manual for exactly how to build and operate the body. DNA is made up of individual building blocks called nucleotides that are joined together in a particular order. The order of the nucleotides serves as a code that tells the cell how to make proteins, which form many of the structures of a cell and carry out most of a cell’s functions.
- Danger signals
Molecules that are released from damaged or dying cells during an infection with a pathogen or physical injury of body tissue that signal to other cells that damage or danger is present. Some danger signals are molecules that are unique to pathogens; these are known as Pathogen-Associated Molecular Patterns (PAMPs). Molecules released by tissues undergoing stress, damage, or abnormal death (e.g., due to inflammation or lack of oxygen) are known as Damage-Associated Molecular Patterns (DAMPs). PAMPs,DAMPs, and other danger signals stimulate dendritic cells, which can activate both innate and adaptive immune responses.
- Delayed type hypersensitivity (DTH)
A strong inflammatory response that can be observed 1-3 days after exposure to a molecule that elicits an immune response (antigen). The response takes time to develop because interactions between immune cells, mainly T cells, macrophages, and dendritic cells, are involved.
- Dendritic cells (DCs)
A type of immune cell that patrols the body looking for cells that are unhealthy or in danger from an invading virus or bacteria. When dendritic cells sense danger, they gather and internally process nearby dying cells and cell debris. The dendritic cells then travel to the lymph nodes where they show evidence of threats (antigens) to T cells – the key active warriors in the immune system’s fight against cancer. If a particular T cell recognizes the antigen, it becomes activated and stimulated to multiply into a T cell army that ultimately attacks the threatening cells. Dendritic cells are a type of antigen-presenting cells and there are many different types of dendritic cells.
- Driver mutation
A change or mutation in the sequence of the DNA of a cell that can lead to or cause cancer. Mutations in DNA can cause changes within a cell, and while most mutations are harmless or will simply cause that cell to die, occasionally, a mutation will give a cell a competitive edge that allows it to thrive and multiply out of control, eventually leading to the development of cancer. These driver mutations usually occur in genes that control a cell’s survival, persistence, and multiplication process. There are hundreds of different genes that can become mutated in a way that causes cancer.
D
- Eosinophil
A type of immune cell that can attack and kill bacteria, cells, and some parasites. It releases toxic materials to attack infected or unhealthy cells, and can be involved in allergy, asthma, or in the destruction of cancer cells. Eosinophils also can act as antigen-presenting cells, can regulate other immune cell functions, and can promote the repair of damaged tissue. Eosinophiles are a type of granulocytes.
- Epidermal growth factor receptor (EGFR)
The EGFR gene provides the instructions to generate the EGFR protein, also called ErbB1 or HER1, which is found on the surface of certain cell types and is involved in sending signals that direct cells to multiply. Cancer-causing mutations in the EGFR gene, including insertions or deletions (addition to or removal of parts of the gene) may turn the EGFR protein on and keep it on and/or produce abnormally high levels of EGFR on the cell surface, causing cells to start to multiply uncontrollably. Mutations in EGFR and abnormally high levels of EGFR on the cell surface have been associated with various cancers, including some lung cancers, colorectal cancers, and head and neck cancers. Therapies called EGFR inhibitors, such as afatinib, dacomitinib, erlotinib, gefitinib, and osimertinib, can help slow down tumor growth. Antibody therapies, including necitumumab (Portrazza), amivantamab (Rybrevant), panitumumab (Vectibix), and cetuximab (Erbitux) may also be used to treat patients with EGFR-positive cancer.
- Epitope
A small part of a larger molecule that is the key identifier of an antigen (an abnormal molecule that is present in cancer cells or molecules from viruses, bacteria, or other threats). Epitopes can be recognized by antibodies, T cells, and B cells. For T cells, epitopes are peptides – fragments of proteins produced by degradation within the cell – that are loaded onto MHC molecules and presented on the surface of other cells. While T cells can only recognize peptides from degraded proteins that are loaded onto MHC molecules as epitopes, B cells and antibodies can recognize epitopes that are part of a whole, undegraded molecule and are not limited to proteins.
E
- Food and Drug Administration (FDA)
The US agency that is responsible for reviewing, monitoring, and regulating food, human and veterinary drugs, biological products, medical devices, cosmetics, and products that emit radiation. For a drug or medical device to be approved for use in the US, the FDA must determine that it provides benefits that outweigh its known and potential risks to the intended population.
F
- Gene
A sequence of nucleotides (the individual building blocks) in DNA that codes for the amino acid sequence of a protein.
- Genetic engineering
The process of altering DNA by adding, removing, or editing segments of the DNA that carry select genes. Genetic engineering can be used to change the characteristics of a cell, its behaviors, or the proteins it produces. Scientists may utilize this process on cells in a laboratory to have them produce proteins like cytokines that can be isolated and used for immunotherapy. They may also use it to enhance cells that will be used for cell therapies, like TCR-engineered T cells or CAR T cells.
- Graft-versus-host disease (GvHD):
A condition that may occur in patients after a bone marrow or stem cell transplant when immune cells of the bone marrow or stem cells from the donor (the ‘graft’) identify the patient’s healthy tissue (‘host’) as foreign and thus, attack the patient’s body. The likelihood of Graft-versus-host disease (GvHD) depends on how closely related the donor and recipient are, with close genetic matches lowering risk of GvHD.There are two types of GvHD:
- Acute GvHD: onset occurs within the first 100 days after transplant. Symptoms usually appear on the skin, in the gastrointestinal tract, and/or in the liver. Treatment typically involves short-term immunosuppression in the form of corticosteroids.
- Chronic GvHD: onset occurs later than 100 days after transplant. Chronic GvHD is considered an autoimmune disorder and can affect any tissue and/or organ. Treatment typically involves long-term immunosuppressant medications.
- Granulocytes
A subset of immune cells including neutrophils, basophils, eosinophils, and mast cells. These cell types contain toxic materials that can be used to kill other cells. The toxic material is stored in small vesicles (granules) within the cell. The vesicles are released in response to a threat to attack the target cells.
G
- HER2 (human epidermal growth factor receptor 2)
A protein that is found on the surface of certain cell types and sends signals that direct cells to multiply. Mutations in the gene that provides the instructions to generate the HER2 protein (also called ERBB2) may cause HER2 to be active all the time and/or to increase the production of HER2, which allows cells to multiply uncontrollably and drives the development of cancer. These mutations occur in about 15-20% of breast cancers (referred to as HER2-positive breast cancers), and can also occur in other cancer types, such as ovarian, stomach, lung, and gastric cancers. HER2-positive cancers may be treated with antibody therapies, including trastuzumab (e.g., Herceptin), pertuzumab (Perjeta), ado-trastuzumab emtansine (Kadcyla), fam-trastuzumab deruxtecan (Enhertu), margetuximab (Margenza), and pertuzumab plus trastuzumab (Phesgo).
- Haematological malignancies
A class of cancers that affect the blood cells, mainly white blood cells (such as leukocytes, including lymphocytes, myeloid cells and plasma cells). Haematological malignancies are categorized by their detection site – the place in the body where the cancer is first found. Leukemias are cancers first detected in the blood, lymphomas are first detected in the lymph nodes or in other lymphatic tissue such as the spleen, and myelomas are first detected in the bone marrow.
- Hematopoietic stem cell
A type of cell that primarily resides in bone marrow (the sponge-like cavity inside of bones) and gives rise to new red blood cells, immune cells, and platelets, which go on to circulate throughout the body.
- Hospice care
Care and services for patients who are in later stages of an incurable illness (such as advanced or metastatic cancer) and/or are nearing the end of their life. In a hospice care setting, a team of professionals (e.g., doctors, nurses, social workers, psychologists, and chaplains) provide mainly palliative care to reduce pain and maximize comfort. Hospice care workers may also help patients and family members or friends of the patient with end-of-life planning, and provide services to address psychological, emotional, social, and spiritual needs. Hospice care can be accessed in a patient’s home, a care facility, or a hospital.
- Human leukocyte antigens (HLA)
A term frequently used interchangeably with MHC. The term HLA describes the human MHC molecules, while the more generic term MHC is also used to describe the MHC molecules in animals.
- Humoral immune response
An immune response involving antibodies.
H
- Immune escape
A term describing cases in which cancer cells can evade elimination by the immune system. Immune escape is part of the immunoediting process by which the immune system can both protect an individual from cancer (even before it becomes clinically detectable) and support tumor growth by promoting the appearance of cancer cells that can survive immune attacks.
- Immune surveillance
A term for the routine monitoring process of the immune system to detect and destroy potential threats to the body, including cancer cells. In some cases, cancer cells are eliminated before they become clinically detectable, and in other cases, immune escape allows cancer cells to evade elimination by the immune system. Immune surveillance and immune escape are parts of the immunoediting process by which the immune system can both protect an individual from cancer and support tumor growth by promoting the appearance of cancer cells that can survive immune attacks.
- Immunoediting
A process by which the immune system can both protect an individual from cancer (even before it becomes clinically detectable) by recognizing and killing cancer cells that contain abnormal molecules (antigens), and at the same time support tumor growth by promoting the survival of those cancer cells that stopped producing the recognized antigens.
- Immunoglobulin (Ig)
A term used to designate the different forms of an antibody (antibody classes). Each antibody form has different properties, and the four most studied classes of antibodies are:
- IgM
An early form of an antibody that is still bound to the surface of a B cell which is just beginning to have the capacity to produce the antibody. - IgG
A form of the antibody that is released from the B cell and can circulate throughout the body to find its target. - IgA
A form of the antibody that interacts with itself to form larger structures. IgA is particularly important to protect against airborne and food-borne threats. - IgE
A form of the antibody that is focused on parasitic threats and is also involved in common allergic reactions.
- IgM
- Immunological tolerance
A situation when the immune system recognizes a cell or a molecule as familiar and so it does not attack it. Immunological tolerance is necessary to prevent the immune system from mistakenly attacking normal cells. Because cancer cells develop from normal cells, they are frequently tolerated by the immune system. One of the main goals of immunotherapy is to identify ways to carefully overcome immunological tolerance without launching an attack against normal cells. In some cases, cancer cells produce molecules that are abnormal (e.g. viral antigens or neoantigens). Abnormal molecules are not protected by immunological tolerance and can stimulate a strong immune response.
- Immunosuppressive
A term used to describe anything that reduces the effectiveness of the immune system in fighting against threats. “Immunosuppressive” may be used to describe something that affects the entire body, like chemotherapy, or something that occurs locally within a tissue, like cancer cells producing molecules to slow down attacking T cells. Some cells (e.g. regulatory T cells) or parts of the immune system itself have immunosuppressive effects on other cells or parts of the immune system to prevent over-reactive immune responses or an immune responses against normal, healthy cells (autoimmune attack).
- Immunotherapy
A treatment designed to alter a patient’s immune response. Cancer immunotherapy aims to produce, maintain, or amplify the immune response to cancer. For autoimmune disease, organ transplantation, or allergy desensitization, the aim is to reduce or suppress the existing immune response.
- Inflammation/inflammatory response
The immune system’s protective response to indications of the presence of harmful pathogens, toxic compounds, or dead, damaged, or abnormal cells, such as cancer cells. The common inflammatory response mechanism includes three steps: 1) local immune cells recognize the harmful stimuli, 2) the cells respond to the infection or injury by activating a series of pathways and releasing messenger molecules (cytokines or chemokines) that alert nearby cells, and 3) Immune cells are recruited to the site. The end goal of the response is to remove the harmful trigger and initiate the healing process.

- Inhibitory receptor
A receptor on an immune cell that inhibits or slows down the activity of the immune cell.
- Innate immune system
One of the two arms of the immune system. The innate immune system includes cells that are always ready for immediate action. These “first responders” are not specific to any particular threat (antigen). Major cell types of the innate immune system include macrophages, dendritic cells, natural killer cells, and granulocytes.
- Interferons (IFN)
A small protein produced and released by an immune cell that acts as a signal and affects other cells. Sometimes, a cell secretes interferons to affect itself. In particular, IFNγ (interferon gamma) plays an important role in the antitumor immune response and is produced by certain cells of the immune system, such as T cells and NK cells. Interferons were originally identified as molecules that “interfere” with a viral infection.
- Interleukin (IL)
A small protein released by white blood cells (leukocytes) that acts as a signal and affects other cells. Sometimes, a cell secretes interleukins to affect itself. Interleukin is an alternate name for cytokine. The term interleukin is used in the naming of many specific cytokines such as interleukin-2 (IL-2).
- Invariant natural killer cells (iNKT) cells
A relatively rare immune cell type with properties of both T cells and natural killer (NK) cells. iNKT recognize a specific class of antigens, and have been observed to respond quickly (within minutes) to potential threats. Due to these unique capacities, iNKT cells are being investigated for their potential to be utilized in cancer immunotherapies.
I
- Leukapheresis
A process by which immune cells are collected from a patient. During leukapheresis, a patient is hooked up to a machine that filters their blood, collecting the immune cells and returning the rest of the blood to the patient. This may be done in order to reduce a high number of white blood cells, to collect immune cells for analysis, or to manufacture a personalized cell therapy, like T cell therapy.
- Leukemia
A type of blood cancer (hematological malignancy) that originates in the blood-forming tissue, such as the bone marrow. The bone marrow then produces a large number of abnormal blood cells (‘blasts’) that enter the bloodstream.
- Leukocyte
A type of immune cell that is made in the bone marrow and found in the blood and lymph tissue. Leukocytes are white blood cells that have a nucleus. These characteristics distinguish leukocytes from the other major types of cells in the blood: red blood cells, which are red and have no nucleus, and platelets, which have no nucleus. Leukocytes include granulocytes (e.g. neutrophils), lymphocytes (T and B cells), and myeloid cells (monocytes, macrophages, and dendritic cells).
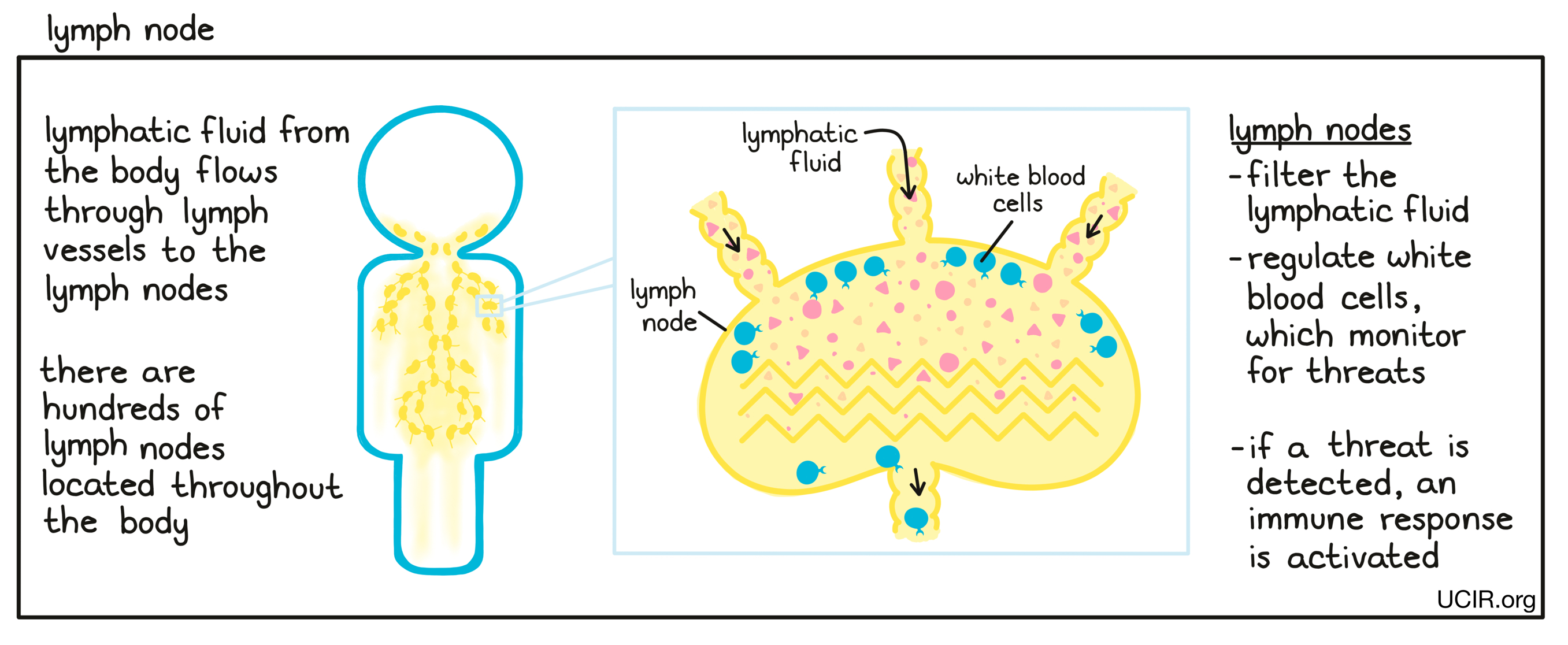
- Lymph node
A small, bean-shaped organ that is part of the immune system. Hundreds of lymph nodes are located throughout the body, and they are connected to each other and to most tissues in the body by lymph vessels. Lymphatic fluid, which flows throughout the body, captures cells, cell debris, and potentially harmful substances from tissues and passes through the lymph nodes. The lymph nodes filter the lymphatic fluid and regulate the interactions of different types of white blood cells (leukocytes) in order to activate an appropriate immune response against infections and diseases such as cancer.
- Lymphocytes
A subset of immune cells including T cells, B cells, and NK cells. These cell types can identify and eliminate molecules and cells that don’t belong (e.g. allergens, such as pollen; virus-infected cells; or cancer cells).
- Lymphodepletion
The partial destruction of lymphocytes in a patient (usually by chemotherapy or irradiation) as a pre-treatment for T cell therapy or other cellular therapies. Decreasing the number of natural immune cells in the body makes room for the newly transferred cells and reduces competition between the cells for resources (such as cytokines) in the blood.
- Lymphoma
A type of blood cancer (hematological malignancy) that starts in blood cells called lymphocytes that are part of the immune system. Lymphoma is often first detected in the lymph nodes or in other lymphatic tissue such as the spleen.
L
- MET
A protein that is found on the surface of certain cell types and sends signals that direct cells to transition (during development), multiply, thrive, and/or form new blood vessels, depending on the setting and on other molecular signals. Mutations in the gene that provides the instructions to generate the MET protein (also called HGFR) may cause MET to be overly active and/or increase the number of MET proteins present on the cell surface, sending excess signals for the cells to thrive and multiply, thus driving the development and progression of cancer. It may also drive changes in cells that allow the cancer to spread to other organs (metastasize). MET mutations may be present in various cancers, including lung, kidney, liver, stomach, breast, and brain cancers. Therapies that inhibit MET, such as crizotinib and cabozantinib, can help slow down tumor growth in MET-positive cancers. Antibody therapy with amivantamab (Rybrevant) is also used to treat MET-positive cancer.
- Macrophage
A type of immune cell that patrols the body and ingests foreign substances, bacteria, unhealthy cells, and cell debris. Macrophages are found throughout the body and are important in normal body repair and wound healing as well as in clearing up cells and cell debris during infection. Macrophages can also function as antigen-presenting cells and can begin immune responses against various threats, including cancer.
In cancer, certain types of macrophages (called “M1”) can help eliminate the tumor, while others (called “M2”) help the tumor grow. Which types of macrophages are present in tumors depends on the complex signals coming from the cancer cells and all the other cells that are part of the tumor (tumor microenvironment).- Major histocompatibility complex (MHC)
A combination of two proteins (a “protein complex”) on the surface of cells that work together to present potential threats to T cells – the key active warriors in the immune system’s fight against cancer.
Antigen-presenting cells (APCs) – a specialized type of immune cells – are able to “eat” dying or unhealthy cells, invading viruses, or bacteria and digest the protein molecules they eat into smaller pieces (peptides). The MHC protein complex is able to bind to some of these peptides and move (loaded with a peptide) to the cell surface where the peptide can be seen by T cells as antigens. If a particular T cell recognizes the antigen, it becomes activated and stimulated to multiply into a T cell army that ultimately attacks the threatening cells. T cells use their T cell receptor to identify the antigen bound to the MHC molecule as evidence of the presence of a threat. Normal cells and cancer cells also routinely break down protein molecules from within the cell into peptides and present them (bound on MHC) to T cells.
There are two different types of MHC – MHC-I and MHC-II – which specialize in presenting peptides to the two different types of T cells (CD8+ T cells and CD4+ T cells, respectively). For both MHC-I and MHC-II, there are multiple MHC genes in each individual person and multiple versions of each gene that are different from one person to another. This diversity means that each person has multiple opportunities to respond to a threat, and that different people can respond in different ways to the same threat.- Mast cell
A type of immune cell found in tissues. Mast cells are coated with a particular subset of antibodies known as IgE. Binding of the IgE on a mast cell to threats that it recognizes activates the mast cell to release various inflammatory molecules including histamines. Depending on the context, mast cells may either hinder or support tumor growth. Mast cells also play a role in blood vessel development and wound healing. Mast cells are a type of granulocytes.
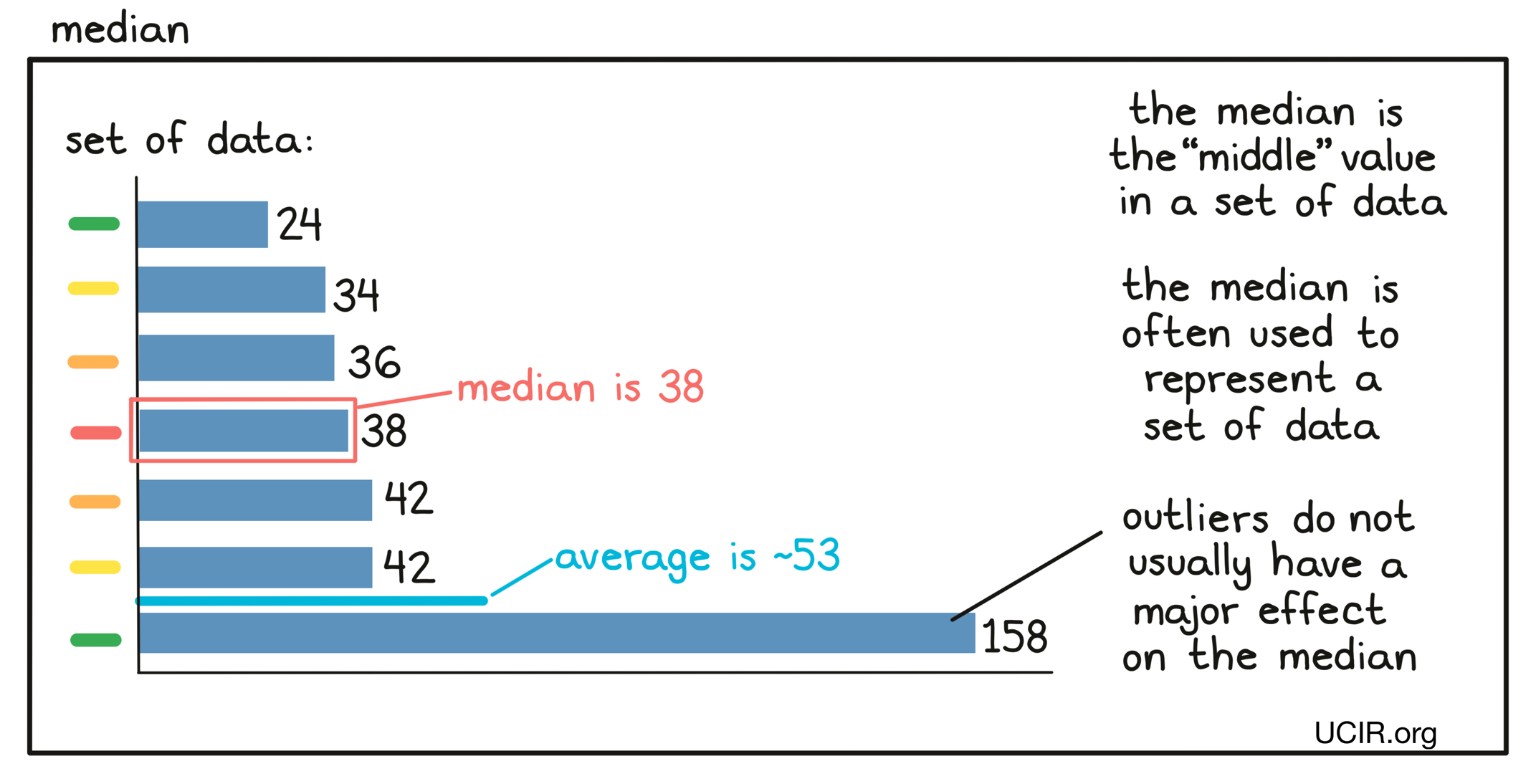
- Median
The middle value in a set of measurements that are arranged in order of increasing magnitude. Essentially, half of the values are equal to or larger than the median, while the other half of the values are equal to or smaller than the median. Unlike the average (also known as the mean), the median does not change much if a few extremely small or extremely large values (outliers) compared to the rest of the data set are present. Sometimes the median and the average are very close to one another; sometimes they are not.
- Median follow-up time
The median length of time that patients who participate in a clinical trial were monitored after initiation or end of treatment. In a clinical trial, patients are usually monitored for a set period of time after they initiate or stop treatment. Patients enter the trial over a broad period of time (usually many months to sometimes multiple years), so that at any particular point in time when physicians want to get a snapshot of the results, individual patients will have been monitored for different periods of time. Physicians use the median follow-up time to characterize the time the whole patient population has been monitored. Essentially, half of the patients will have been monitored for a time equal to or longer than the median follow-up time, while half of the patients will have been monitored for a time equal to or shorter than the median follow-up time.
For some clinical trials the median follow-up time might be shorter than the median time to some effect (such as disease worsening) for a treatment group. The median follow-up time is calculated based on all patients on a trial regardless of treatment group, whereas the median time to a response is calculated only based on those patients in that treatment group. For example, if the disease progresses rapidly in one of the treatment groups and those patients leave the trial for other therapies, their follow-up time stops accumulating.
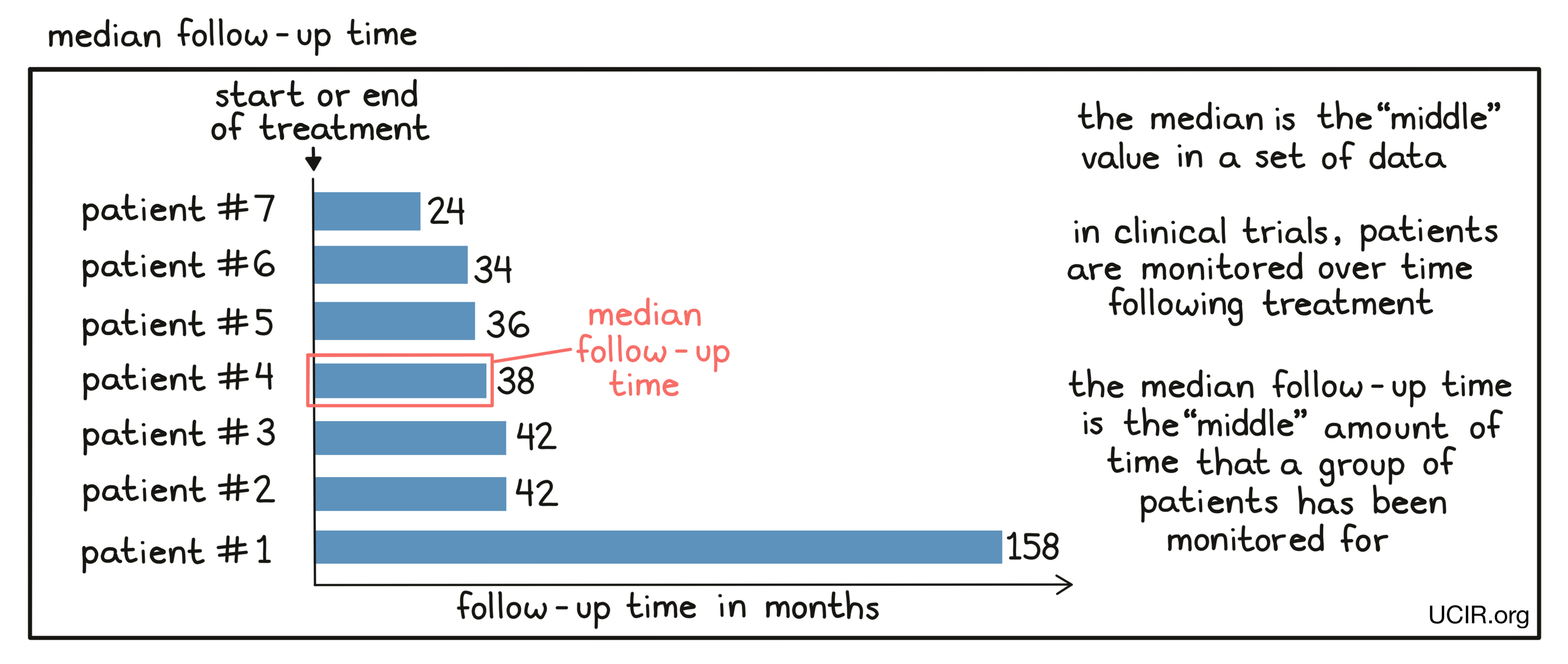
- Memory cell
A long-lived B cell or T cell that has previously recognized a threat (antigen) and “remembers” the antigen. When the same threat appears again, the memory cells are ready to produce a much faster immune response and eliminate the threat.
- Metastatic
A term used to describe cancer that has spread from its original location to somewhere else in the body. For example, breast cancer that has spread to the lungs is considered metastatic breast cancer. In metastatic cancer, cancer cells travel through the blood or lymphatic system to find new tissues to settle into. Cancer that is metastatic is typically more advanced and more difficult to treat than cancer that has not spread from its original location.
- Microbiome
The collection of microorganisms (primarily bacteria) living in an individual, particularly in the gut. The microbiome can have a strong impact on various bodily functions, including the function of the immune system. Altering the microbiome has been studied as a strategy for improving cancer immunotherapy.
- Microsatellite instability-high (MSI-H) cancer
A type of cancer that is characterized by mutations (changes) in microsatellites, which are short, repeated sequences of DNA. Microsatellites are particularly difficult sections of DNA for cells to accurately copy when they multiply, and therefore MSI-H cancers are prone to produce mutations in these DNA regions. MSI-H cancers are most common in colorectal cancer, other types of gastrointestinal cancer, and endometrial cancer, but may also be found in many other types of cancer.
- Mismatch repair-deficient (dMMR) cancer
A type of cancer that is characterized by the accumulation of many changes (mutations) in the DNA, which stems from defects in the ability of the cancer cells to correct mistakes made when a cell copies its DNA for multiplication. Mismatch repair-deficiency is most common in colorectal cancer, other types of gastrointestinal cancer, and endometrial cancer, but may also be found in cancers of the breast, prostate, bladder, and thyroid.
- Monocyte
A type of immune cell that circulates in the blood. When a monocyte leaves the blood and migrates into a tissue, it matures into a macrophage, and occasionally into a dendritic cell, depending on the signals it receives.
- Mouse model
Mice that are bred for research in biology or disease. Mice share many important biological, physiological, and genetic characteristics with humans, and they can be easily bred or even genetically engineered to model particular traits or diseases.
- Mutation
A change in the sequence of the DNA of an individual. DNA is a string of 4 different molecules (“nucleotides”) – adenine (A), cytosine (C), guanine (G), and thymine (T). The order of these nucleotides is a “code” which contains all the genetic information of an individual. The DNA encodes the proteins made by the cell, determines whether and how much of each protein is made, and contains multiple other types of information needed by the cell to function.
Mutations are changes in the “code“ (for example, the replacement of a C by a T). Some mutations change how cells control their growth and may result in changing a normal cell into a cancer cell. Some mutations change the proteins encoded by the DNA (neoantigens).- Myeloid cells
One of the two major branches of immune cells, along with lymphocytes. Myeloid cells come from the bone marrow and give rise to many types of immune cells, including neutrophils, basophils, eosinophils, mast cells, and monocytes. Monocytes can then further give rise to macrophages and a certain type of dendritic cell. In some cases, the term “myeloid cells” might be used as a more general term to describe all of the cell types that arise from myeloid cells.
- Myeloma
A type of blood cancer (hematological malignancy) that arises in plasma cells (white blood cells that produce antibodies) in the bone marrow.
M
- Nanoparticle
Particles that are between 1 and 100 nanometers in diameter (for comparison, an average sheet of paper is about 100,000 nanometers thick). They can be made from many different materials for many different uses. In biomedical applications, nanoparticles are often used to deliver drugs to specific tissues or as contrast agents for medical imaging. Biomedical nanoparticles are often made of lipid molecules, which encase and protect a drug they carry.
- Natural killer (NK) cell
A type of immune cell that circulates throughout the body and senses signals which indicate that other cells are unhealthy or infected by a virus. NK cells quickly kill such cells and can also recruit other immune cells to begin a more thorough immune response. NK cells have a variety of molecules on their surface that help them to distinguish abnormal cells from normal, healthy cells.
- Natural killer T cells (NKT cells)
A type of immune cell with features of both T cells and NK cells. Like T cells, NKT cells have T cell receptors (TCRs) that can recognize specific targets, and like NK cells, NKT cells can sense more general signals that indicate that other nearby cells are unhealthy. NKT cells are much less common than either NK cells or T cells, however, they can be very effective in killing cancer cells.
- Neoadjuvant therapy
A therapy that is given before the main therapy, usually when the main therapy is surgery. For example, if a patient is going to undergo surgery to completely remove a tumor, they may receive a therapy beforehand in order to reduce the size of the tumor before surgery. It has been observed that neoadjuvant therapies frequently trigger an immune response to cancer, so after a tumor is removed, the immune system can help to destroy any remaining cancer cells. Many different types of therapy can be used as a neoadjuvant therapy, including immunotherapy.
- Neoantigen
An abnormal protein found in cancer cells due to a change (mutation) in the DNA of a gene. Genes contain the information (“genetic code”) that determines the order of amino acids in each protein made by the cell. Some changes in the DNA of a gene cause changes in the amino acid order of the protein. Such altered protein can be recognized by immune cells (T cells and B cells) as abnormal and thus may identify cancer cells for destruction. Mutations that occur in the DNA of a cancer cell are different for each individual patient, and the same mutation is rarely found in other patients.
- Neoantigen vaccine
A type of cancer vaccine that is based on DNA changes (mutations) found in the genes of cancer cells of a patient. Genes contain the information (“genetic code”) that determines the order of amino acids in each protein made by the cell. Some changes in the DNA of a gene cause changes in the amino acid order of the protein. Such altered protein can be recognized by immune cells (T cells and B cells) as abnormal and thus may identify cancer cells for destruction. Because most mutations in the DNA of a cancer cell are different for each individual patient, most neoantigen vaccines are personal – designed and used only for a single patient.
- Neoplasm
An accumulation of large numbers of abnormal cells that has no purposeful function and is composed of cells that multiply more than they should or do not die when they should. Neoplasms may be benign (not cancerous) or malignant (cancerous). Benign neoplasms are usually slow-growing and may push nearby tissue aside, but do not spread into the surrounding tissue and remain at their site of origin. Malignant neoplasms (cancers) can spread into the surrounding tissues and invade other parts of the body via the bloodstream or lymphatic system in a process known as metastasis. Neoplasms that form a mass of tissue are also called tumors.
- Neutrophil
A type of immune cell that has the ability to ingest (“eat”) unhealthy cells or cell debris (phagocyte). Neutrophils can rapidly engulf cells that are coated with antibodies or components of the complement system, as well as damaged cells or cellular debris. Neutrophils are a type of granulocytes.
- Non-small vs. small cell lung cancer
There are multiple types of lung cancer including:
Non-small cell lung cancer
Non-small cell lung cancer is the most common kind of lung cancer, which includes large cell carcinoma, squamous cell carcinoma, and adenocarcinoma.Small cell lung cancer
A fast-growing cancer that arises in tissues of the lung. Under the microscope, the cancer cells look small, oval, and oat-like.- Non-squamous vs. squamous cell lung cancer
Non-squamous cell lung cancer
A type of non-small cell lung cancer that is either a large cell carcinoma or adenocarcinoma.Squamous cell lung cancer
A type of non-small cell lung cancer that arises from squamous cells, which are found on the outer surface of the skin, as well as in the respiratory and digestive tracts and the lining of hollow organs (such as kidney, bladder, and uterus). Squamous cells are flat cells that look like fish scales when seen under a microscope.
N
- Oncolytic virus
A virus that preferentially infects and destroys cancer cells. When an oncolytic virus infects a cancer cell, it stimulates the immune system, and when it kills the cancer cell, the cancer cell releases its contents, which further stimulates an antitumor immune response. Oncolytic viruses are used as a cancer therapy alone or in combination with other immunotherapy drugs.
O
- PD-1
A protein molecule on the surface of activated T cells that can bind to a molecule called PD-L1 on the surface of other cells. When PD-1 on T cells binds to PD-L1, T cells become “exhausted” and unable to perform their killing functions. Cancer cells often produce extra PD-L1 (or cause other cells in the tumor to produce PD-L1) to tire out attacking T cells. Using antibodies to block binding between PD-1 and PD-L1 is a common strategy in immunotherapy (checkpoint blockade) to prevent T cell exhaustion.
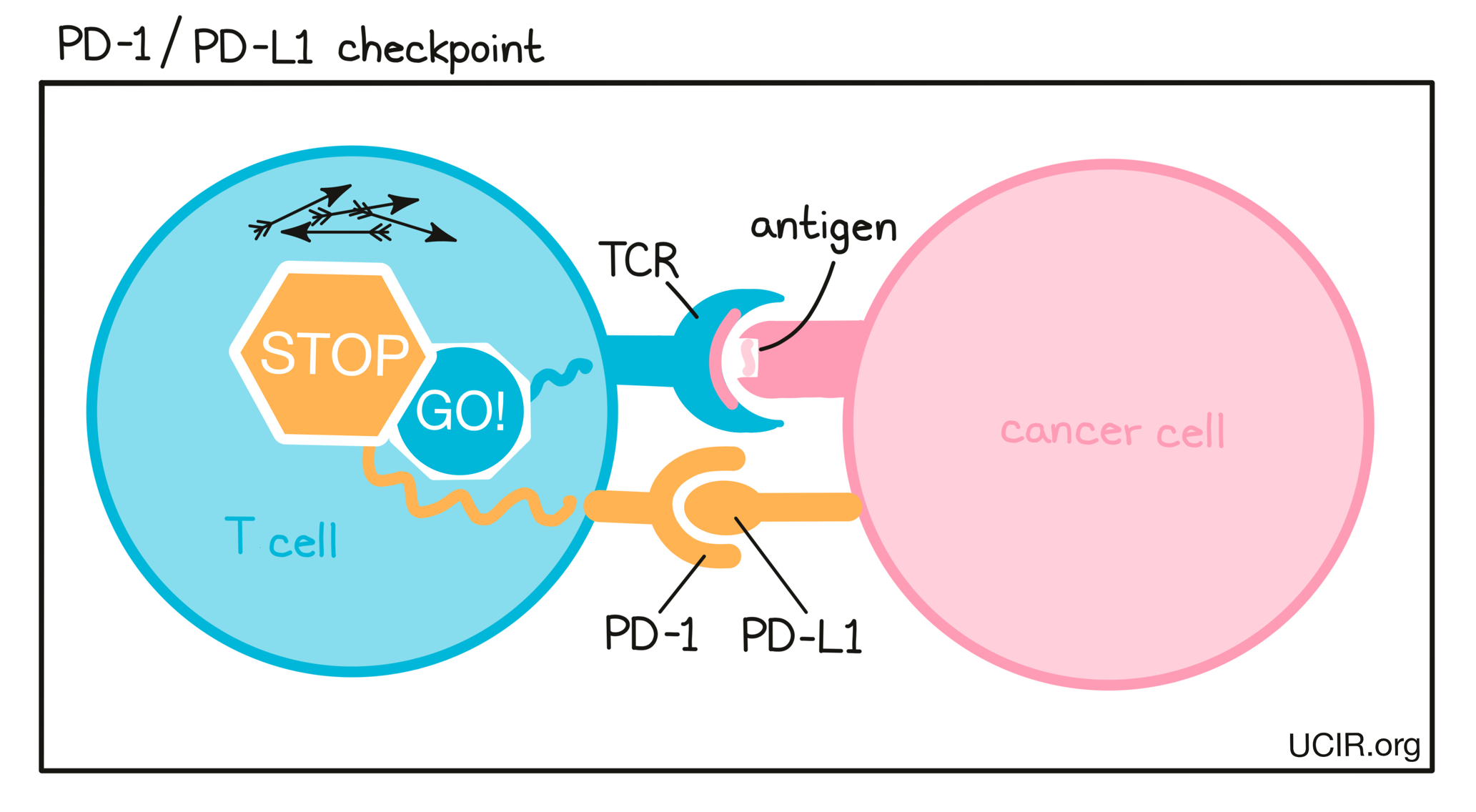
- PD-L1
A protein molecule on the surface of cancer cells (and some other cells in a tumor) that binds to PD-1 on the surface of attacking T cells. When PD-L1 binds to PD-1 on T cells, T cells become “exhausted” and unable to perform their killing functions. Tumors often produce extra PD-L1 to tire out attacking T cells. Using antibodies to block binding between PD-L1 and PD-1 is a common strategy in immunotherapy (checkpoint blockade) to prevent T cell exhaustion.

- Palliative care
A treatment or therapy used to improve quality of life by reducing or controlling the physical or emotional challenges of a disease. In patients with cancer, palliative care can be given at any stage of disease and in addition to cancer treatment.
- Pathogen
An organism such as a bacterium, virus, or fungus that can cause disease in the host it infects.
- Peptide
A type of molecule made up of individual building blocks (each building block is one of 20 different amino acids) joined together in a particular order. Peptides are smaller (shorter) than proteins.
- Phagocyte
A cell that has the ability to ingest (“eat”) unhealthy cells or cell debris. There are many different types of phagocytes, each with specialized functions and names. Two types of phagocytes are especially important for antitumor immunity: macrophages and dendritic cells. Both function as antigen-presenting cells and can initiate a T cell response against cancer cells.
- Philadelphia chromosome
A mutated form of chromosome 22. In cells, DNA is packaged around proteins into structures called chromosomes, like yarn wrapped into an organized twist. Occasionally, these chromosomal structures become damaged or accidentally linked, and in the effort to repair the damage, two chromosomes exchange DNA. The Philadelphia chromosome mutation arises when chromosome 9 and chromosome 22 swap DNA, leaving chromosome 22 shorter than normal, with less DNA packaged into it. This event typically occurs in just one cell in the bone marrow, but as this cell multiplies, its daughter cells inherit the mutated form of chromosome 22. The accumulation of mutated cells may cause chronic myeloid leukemia (CML). Cells carrying the Philadelphia chromosome can also be found in acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML).
- Plasma cell
A type of white blood cell (leukocyte) that produces large amounts of a specific antibody in response to the encounter with a specific molecule or part of a molecule that is recognized as a threat (antigen) to the body. Plasma cells develop from B cells that have been activated.
- Plasmacytoid dendritic cells (pDC)
A type of immune cell that can function as an antigen-presenting cell by capturing damaged, infected, or abnormal cells and cell debris from unhealthy tissues, migrating to lymph nodes, and presenting evidence of the potential threat to T cells – the key active warriors in the immune system’s fight against cancer – and B cells. Plasmacytoid dendritic cells also play a major role in producing large quantities of the cytokine type I interferon (IFN), which is required for the stimulation of a strong immune response.
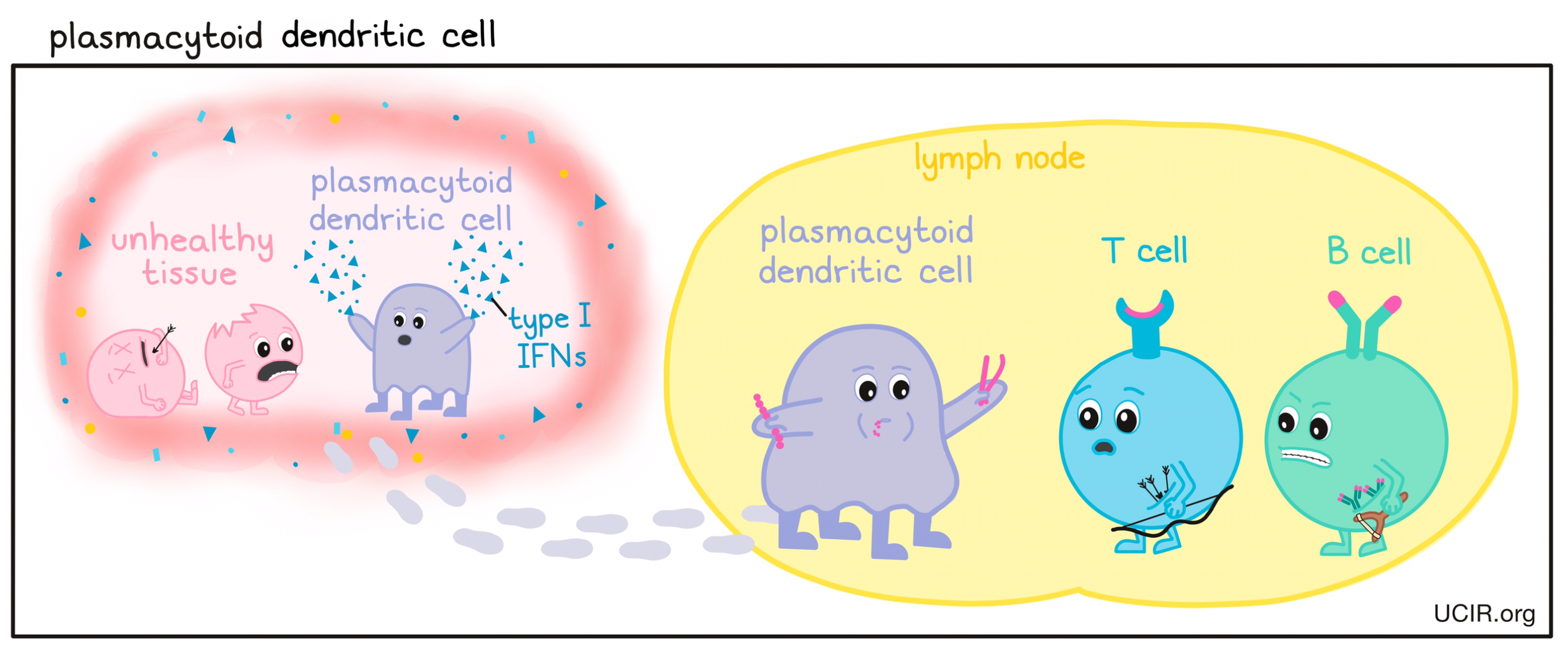
- Platelets
Cellular fragments (~1/5 the size of a red blood cell) found in the blood and spleen that circulate throughout the body scanning the blood vessels for damage. When damage is found, platelets promote blood clot formation and healing. Platelets are also able to recognize and bind to pathogens, damaged cells, or cells coated in antibodies, and secrete molecules that recruit and activate immune cells and stimulate inflammation.
- Platinum-based chemotherapy
A type of chemotherapy that contains the chemical platinum. These types of drugs are used to treat almost half of people receiving chemotherapy for cancer. Commonly prescribed examples of platinum-based chemotherapies include cisplatin, carboplatin, and oxaliplatin.
- Progression
A continued growth or worsening of cancer towards a more advanced state. Progression occurs when a patient’s natural immune response to cancer is not strong enough, or when treatment is not sufficient. Progression can happen at different rates for different cancers. Slowing or halting progression is a measure of success in the treatment of cancer.
- Proteasome inhibitor
A type of drug used to treat cancer by blocking the action of proteasomes – structures within the cell that break down proteins. Inhibiting proteasomes may prevent cancer cells from breaking down proteins that signal that it is time to die, forcing the cancer cells to follow normal cellular commands that they might otherwise ignore.
- Protein
A type of molecule made up of individual building blocks (each building block is one of 20 different amino acids) joined together in a particular order. The order of amino acids determines what role the protein has in the body and is defined by a gene in the DNA of the individual.
P
- RAS gene family
RAS genes are a small family of genes that, when mutated, are involved in causing various types of cancer. RAS genes code for RAS proteins, which tell the cell to be more active or to multiply. In normal cells, RAS genes can be turned on and off as needed, but certain mutations in RAS genes can cause them to become permanently activated, leading to constant cell activation and multiplication. This can cause cells to grow out of control and become cancerous.
- RNA or messenger RNA
A type of molecule that is a copy of the genetic instructions from DNA and carries those instructions to other parts of the cell to be carried out. The instructions are for making proteins, which make up many of the structures within cells and carry out most of a cell’s functions.
- Radiation
A cancer treatment that uses high doses of radiation to kill cancer cells and shrink tumors. Radiation may come from outside of the body, using a machine that delivers high-energy beams, or it may involve placing radioactive material inside of the body. Radiation interferes with how cells grow and divide, and while both healthy and cancerous cells are damaged by radiation, cancer cells are more strongly affected, and normal cells can often recover.
- Receptor
A molecule on the surface of, or inside of a cell that binds to one or more specific signaling molecules, which can come from the cell’s surroundings or from within the cell. When a receptor senses a stimulus, it causes the cell to respond accordingly. Receptors play an important role in how cells sense their surroundings and communicate with one another.
- Recurrence
A reemergence of cancer after a patient has a complete response to therapy, meaning that the cancer was reduced to levels that were undetectable. If even a single cancer cell remains in the body after successful treatment, it can lead to a recurrence of cancer. Recurrence is a form of relapse, and these terms are often used interchangeably.
- Refractory cancer
Cancer that is unresponsive to treatment. A cancer may be considered refractory if it fails to respond to treatment entirely, or if it initially responds to treatment, but then stops responding.
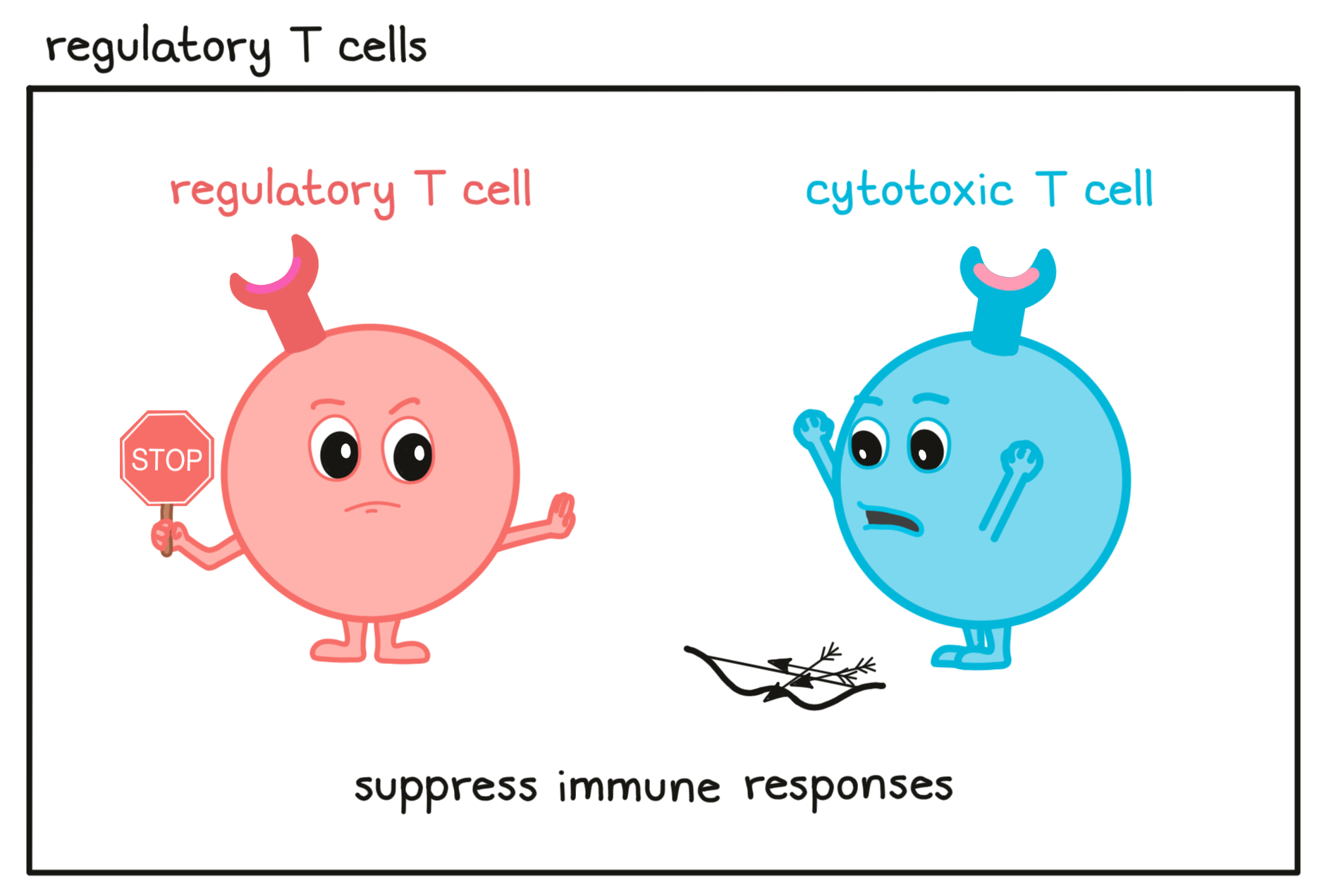
- Regulatory T cell
A type of T cell that is found throughout the body and helps to dampen or block the activity of other immune cells to prevent overreactions. Eliminating regulatory T cells or reducing their suppressive activity is an important strategy in cancer immunotherapy.
- Relapse
A reemergence or continued growth (progression) of cancer after a temporary response to treatment. During a response, the cancer goes away, shinks, or stops growing. During relapse, it comes back or starts growing again. Relapsed cancer has found a way to escape from the therapy that was initially effective, meaning that the therapy that was initially effective against the cancer may not be effective against the relapsed cancer.
- Remission
A temporary or permanent decrease in the signs and symptoms of cancer. Remission can be partial or complete. In a complete remission, all of the signs and symptoms of cancer have disappeared.
R
- Sarcoma
A type of cancer that originates in the bones or in soft tissues such as cartilage, muscle, fat, blood vessels, cells that cover and protect nerves, tendons, and the lining of joints or bones. There are more than 70 known types of sarcoma.
- Signaling pathway
A group of molecules outside and inside of a cell that work together to control one or more cell functions, such as telling the cell to migrate, to multiply, or to die. In this cell communication process, cells receive information from their environment or from elsewhere in the cell via receptor molecules. After the first molecule in a signaling pathway receives a signal, it alters the activity of another molecule or molecules, which in turn can alter the activity of another molecule or molecules, and so on, until the cell function involved is carried out. In some cases, more than one cell function can be affected. This complex signaling cascade offers multiple opportunities for regulation, amplification/dampening, or fine-tuning of the cell’s function. In cancer cells, many signaling pathways are altered and constantly active, often leading to uncontrolled cell multiplication.
- Small vs. non-small cell lung cancer
There are multiple types of lung cancer including:
Small cell lung cancer
A fast-growing cancer that arises in tissues of the lung. Under the microscope, the cancer cells look small, oval, and oat-like.Non-small cell lung cancer
Non-small cell lung cancer is the most common kind of lung cancer, which includes large cell carcinoma,squamous cell carcinoma, and adenocarcinoma.- Solid tumors
An abnormal collection of cells that form a mass of tissue and may be cancerous (malignant) or not cancerous (benign). Solid tumors are named for the type of cells that compose them, such as carcinomas (formed by epithelial cells that line organs or make up the skin), melanomas (formed from melanocytes present in the skin) or sarcomas (formed by cells in the bones, fat, muscle, nerves or other).
- Squamous cell carcinoma
A type of cancer that arises from squamous cells, which are found on the outer surface of the skin, as well as in the respiratory and digestive tracts and in the lining of hollow organs (such as the kidneys, bladder, and uterus). Squamous cells are flat cells that look like fish scales when seen under a microscope.
- Squamous cell vs. non-squamous cell lung cancer
Squamous cell lung cancer:
A type of non-small cell lung cancer that arises from squamous cells, which are found on the outer surface of the skin, as well as in the respiratory and digestive tracts and the lining of hollow organs (such as kidney, bladder, and uterus). Squamous cells are flat cells that look like fish scales when seen under a microscope.Non-squamous cell lung cancer:
A type of non-small cell lung cancer that is either a large cell carcinoma or adenocarcinoma.- Stem cell transplantation
A procedure used to treat certain types of cancer, like multiple myeloma or leukemia, in which the patient is first treated with high doses of chemotherapy (and in some cases radiation) to destroy cancer cells that reside in the blood or bone marrow. However, this treatment also kills the patient's normal blood cells and their hematopoietic stem cells, a type of cell which can multiply and form all the different types of cells normally found in the blood. These blood-forming cells can then be replaced with the patient’s own healthy stem cells, which were collected and saved before treatment (autologous), or stem cells from a donor (allogeneic).
- Systemic
Occurring throughout the body. A systemic treatment is one that enters the bloodstream and travels to all parts of the body. A systemic reaction is one that affects the body as a whole, rather than being contained to particular locations.
S
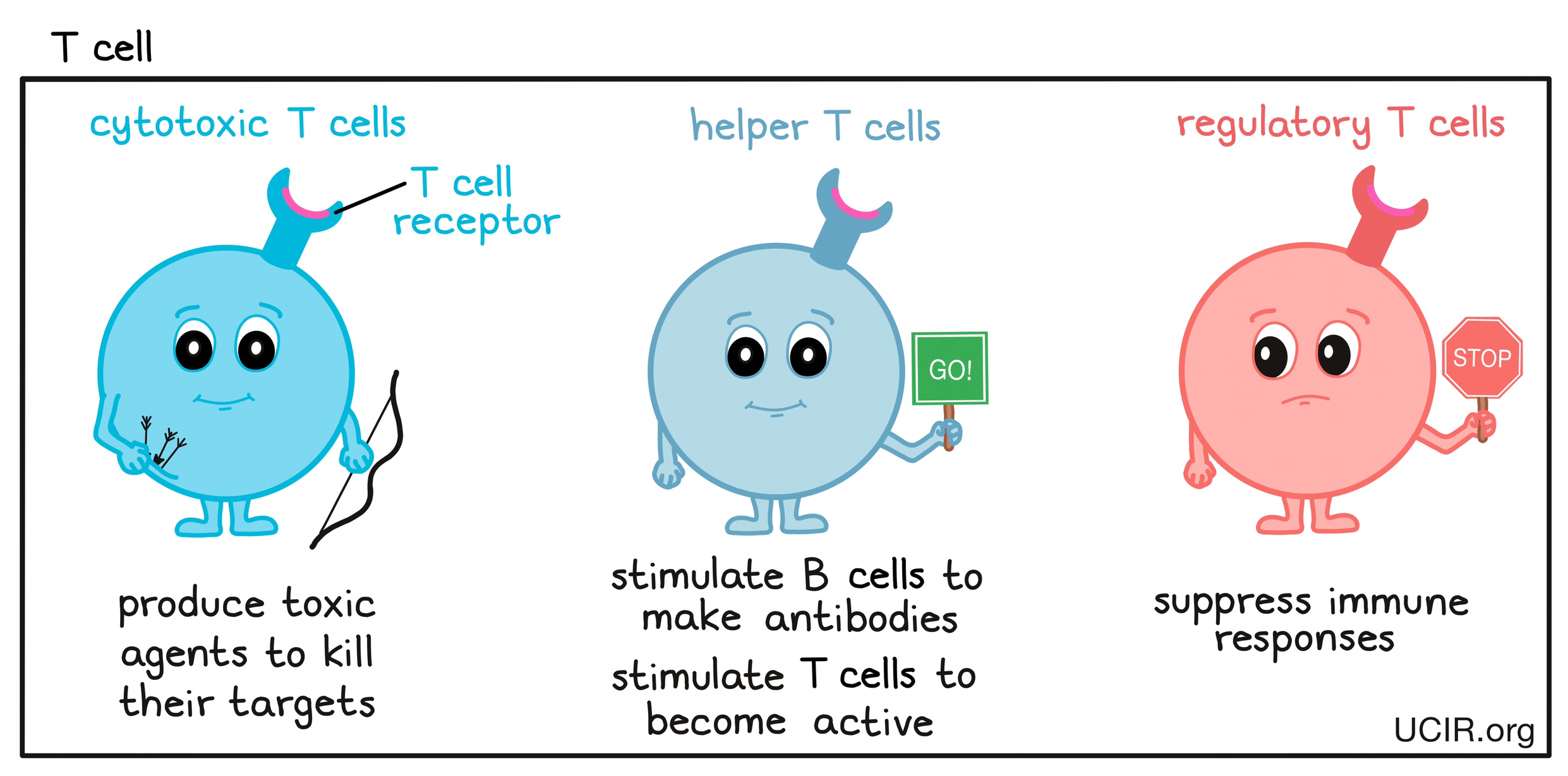
- T cell
A type of immune cell that is the key active warrior in the immune system’s fight against cancer. T cells are first trained and stimulated in the lymph node to recognize cells that don’t belong, and then circulate throughout the body. T cells use structures on their surface called T cell receptors (TCRs), which can very specifically identify unhealthy or threatening target cells. When a threat is detected, T cells multiply into a T cell army that hunts down and kills the threatening cells.
There are different types of T cells:- cytotoxic T cells, which produce toxic agents to kill their targets;
- helper T cells, which stimulate B cells to make antibodies against the targets and stimulate cytotoxic T cells to be active;
- regulatory T cells, which suppress immune responses to keep the immune system in check and prevent harmful overreactions.
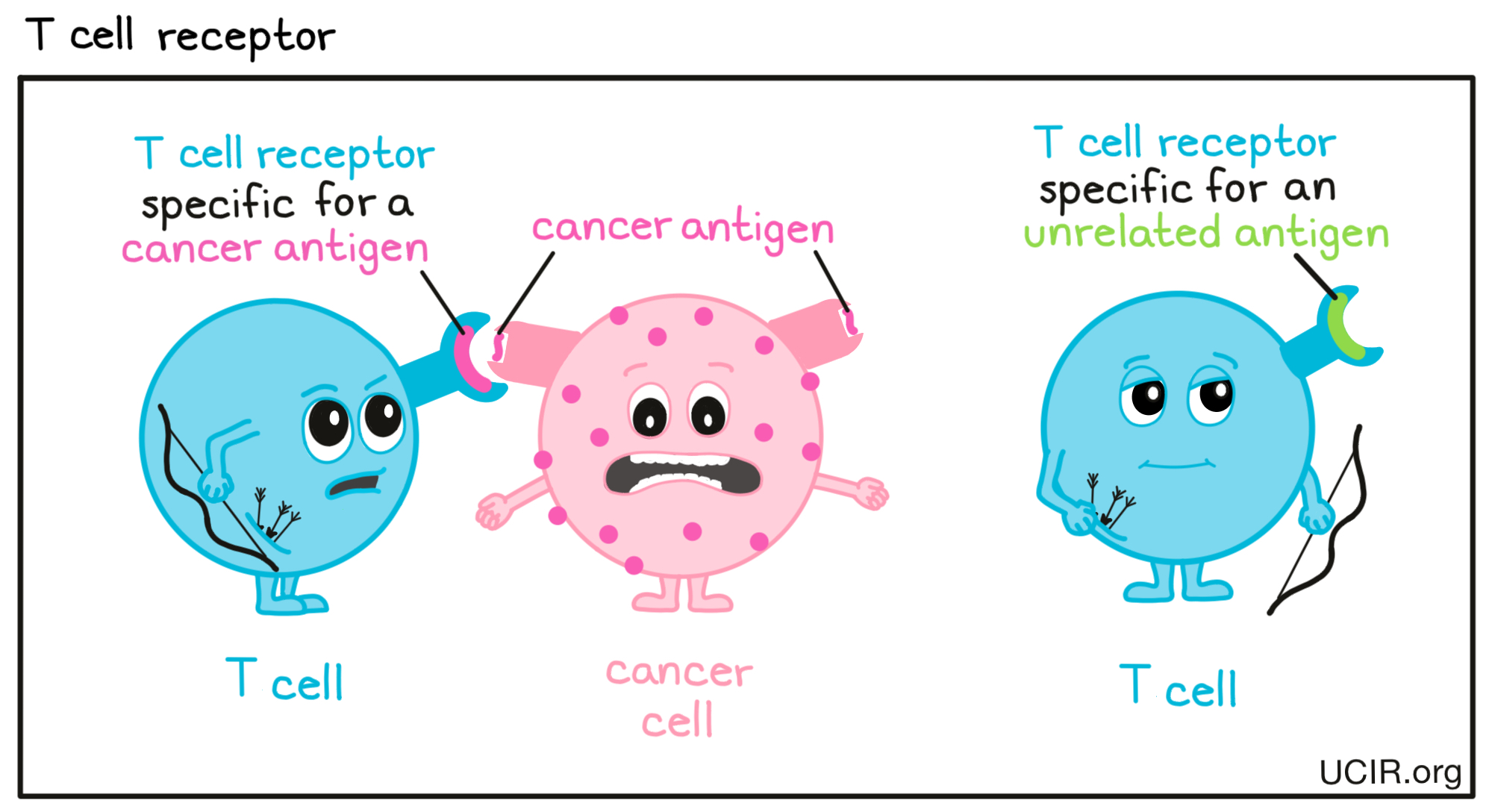
- T cell receptor (TCR)
A structure on the surface of T cells that can very specifically identify target molecules on unhealthy or threatening cells. Each individual T cell is “trained” to target one particular molecule (a “target antigen”). In the case of cancer cells, these would be called “cancer antigens”. The T cell receptor (TCR) and its target antigen fit together like a lock and key. T cells throughout the body use their TCRs to patrol the surface of other cells. When a T cell detects its target antigen, it will multiply into a massive T cell army that hunts down and kills any cells that contain the target antigen.
- Treatment cycles
The basic schedule for a treatment, including the schedule for the treatment itself and the schedule for periods of no treatment (usually called periods of rest). For example, if a treatment needs to be administered every day for one week, followed by three weeks of rest, this 4-week regimen would be referred to as one treatment cycle. This term is usually used when a treatment is going to be repeated.
- Tumor
An abnormal mass of tissue that has no purposeful function and is formed when cells multiply more than they should or do not die when they should. Tumors may be benign (not cancerous) or malignant (cancerous). Benign tumors are usually slow-growing and may push the tissue aside, but do not spread into the tissue surrounding the tumor cells, and remain at their site of origin. Malignant tumors (cancers) can grow into surrounding tissues or spread to other parts of the body via the bloodstream or lymphatic system in a process known as metastasis. Tumors are a type of neoplasm that has formed a solid mass.
- Tumor microenvironment (TME)
A term describing all the parts of a tumor including:
- the cancer cells;
- other cells, such as immune cells and non-immune cells, that might be normally found in the affected tissue or that get attracted to the tumor by the cancer cells;
- blood vessels;
- small protein molecules produced and released by the various cell types present in the tumor, which act as signals: for example, cytokines or chemokines can affect other cells, while growth factors can support cancer cells;
- molecules that fill the space between cells and provide a firm structural scaffold in which cells reside and move (“extracellular matrix”).
The tumor microenvironment can affect cancer cells, and cancer cells can change the tumor microenvironment. Cancer cells often produce factors that make the tumor microenvironment supportive for themselves, but harsh and suppressive for immune cells that could attack the cancer cells. Overcoming the immunosuppressive tumor microenvironment is a key goal in many immunotherapies.
- Tumor-associated antigen
A molecule or part of a molecule that is found in cancer cells and can be recognized by immune cells (T cells or B cells) as abnormal or out of the ordinary. Tumor-associated antigens arise when DNA mutations cause cancer cells to produce molecules at unusually high levels, molecules that are typically only found in specialized cell types (such as egg or sperm cells), or molecules that are not typically produced in adults. Any of these may raise a red flag to the immune system that something is not right.
T
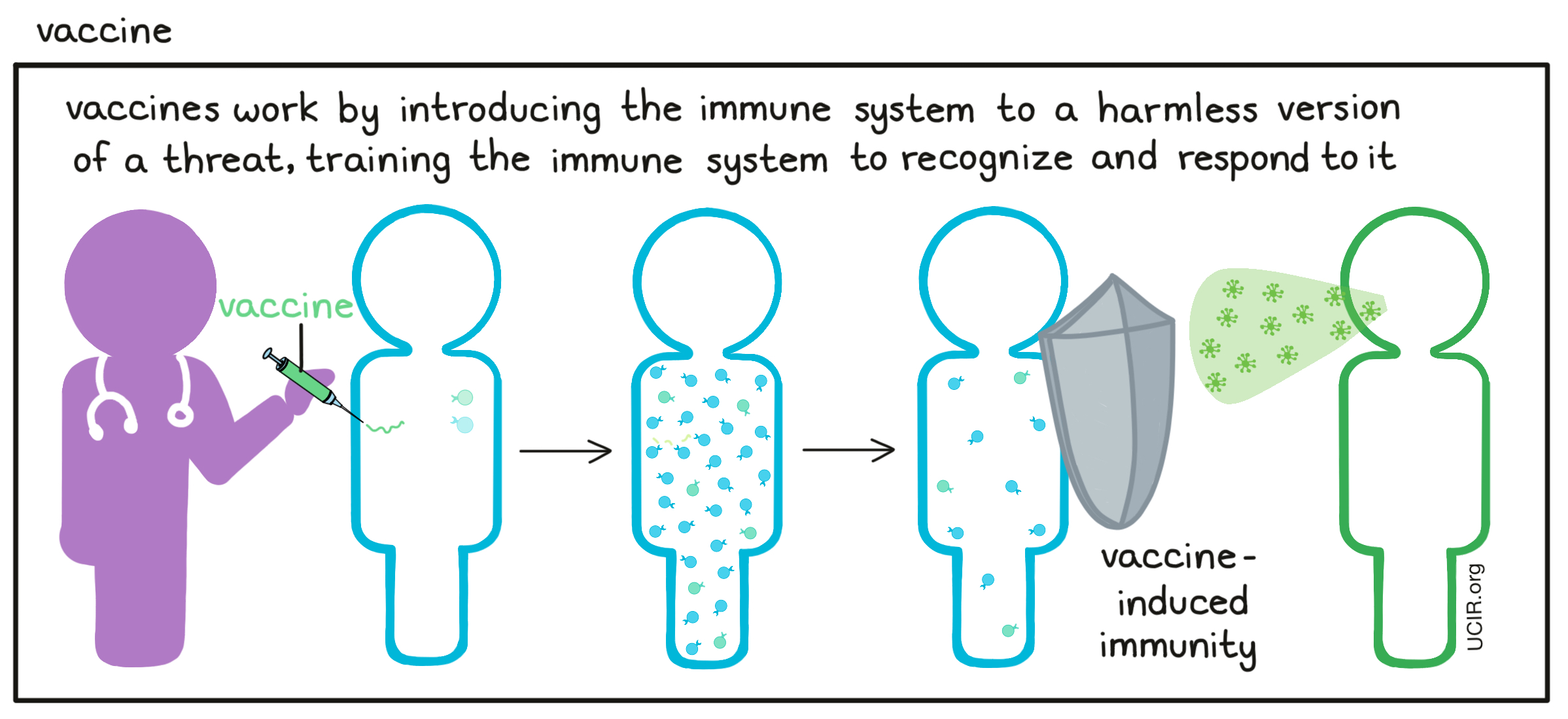
- Vaccine
A type of immunotherapy that trains the immune system to recognize and respond to particular threats, such as viruses, bacteria, or cancer cells. Vaccines introduce harmless versions or representations of the threats in a way that stimulates an immune response against it. Once the vaccine has cleared from the body and the immune response has settled down, some immune cells remain as “memory” cells that patrol the body. If the same threat is ever encountered again, these memory cells can spring into action, protecting the body and preventing severe disease. Some vaccines also include an additional substance, an adjuvant, to signal danger to the immune system and encourage stronger immune activation. Learn more about cancer vaccines here.
A molecule that is derived from a virus and can be recognized by the immune system as “foreign”, something that does not belong in the body. Viral antigens may stimulate an immune response involving T cells or B cells. Some cancers are caused by a virus, and therefore produce viral antigens that the immune system can target.