How is this drug name pronounced?
Cetuximab: seh-TUK-sih-mab
Erbitux: ER-bih-tux
What cancer(s) does this drug treat?
Head and neck squamous cell cancer
Cetuximab is approved for:
- Patients with head and neck squamous cell cancer that has spread to nearby tissue or lymph nodes. In such cases, Erbitux is used in combination with radiation therapy.
- Patients with head and neck squamous cell cancer that has come back after previous treatment or has spread to nearby tissue or lymph nodes or other parts of the body. In such cases, Erbitux is used in combination with platinum-based chemotherapy and fluorouracil.
- Patients with previously treated head and neck squamous cell cancer that has grown or spread to other parts of the body, who have received treatment with platinum-based chemotherapy, and the disease kept getting worse. In such cases, Erbitux is used alone.
Colorectal cancer
Cetuximab is approved for:
-
Patients with colon or rectal cancer that has spread to other parts of the body, and whose cancer has normal (“wild-type”) copies of the KRAS gene and tests positive for the EGFR protein. In such cases, Erbitux is used
- in combination with the chemotherapeutics irinotecan, fluorouracil, and leucovorin as a first treatment.
- in combination with irinotecan in patients who have been previously treated with irinotecan-based chemotherapy and the treatment did not work or stopped working.
- alone in patients who have been previously treated with oxaliplatin and irinotecan chemotherapy and the treatment did not work or stopped working, or who cannot be treated with irinotecan.
-
Patients with previously treated colon or rectal cancer whose cancer tested positive for the BRAF V600E mutation. In such cases, Erbitux is used in combination with encorafenib.
Limitations of use:
Age: The safety and efficacy of Erbitux in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Erbitux may impair fertility in women. Erbitux may cause harm to the fetus and is not recommended for use during pregnancy. Women are advised to use contraception during treatment with Erbitux and for at least 2 months after the last dose of Erbitux. The risks associated with Erbitux during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Erbitux and for at least 2 months after the last dose of Erbitux.
Exclusions: Erbitux should not be administered to patients with colorectal cancers that have mutations in the RAS genes (including KRAS), or when the mutation status of the RAS genes is unknown. A triple combination of cisplatin, Erbitux and radiation therapy should not be administered.
What type of immunotherapy is this?
- Cell growth inhibitor
How does this drug work?
- Target: epidermal growth factor receptor (EGFR)
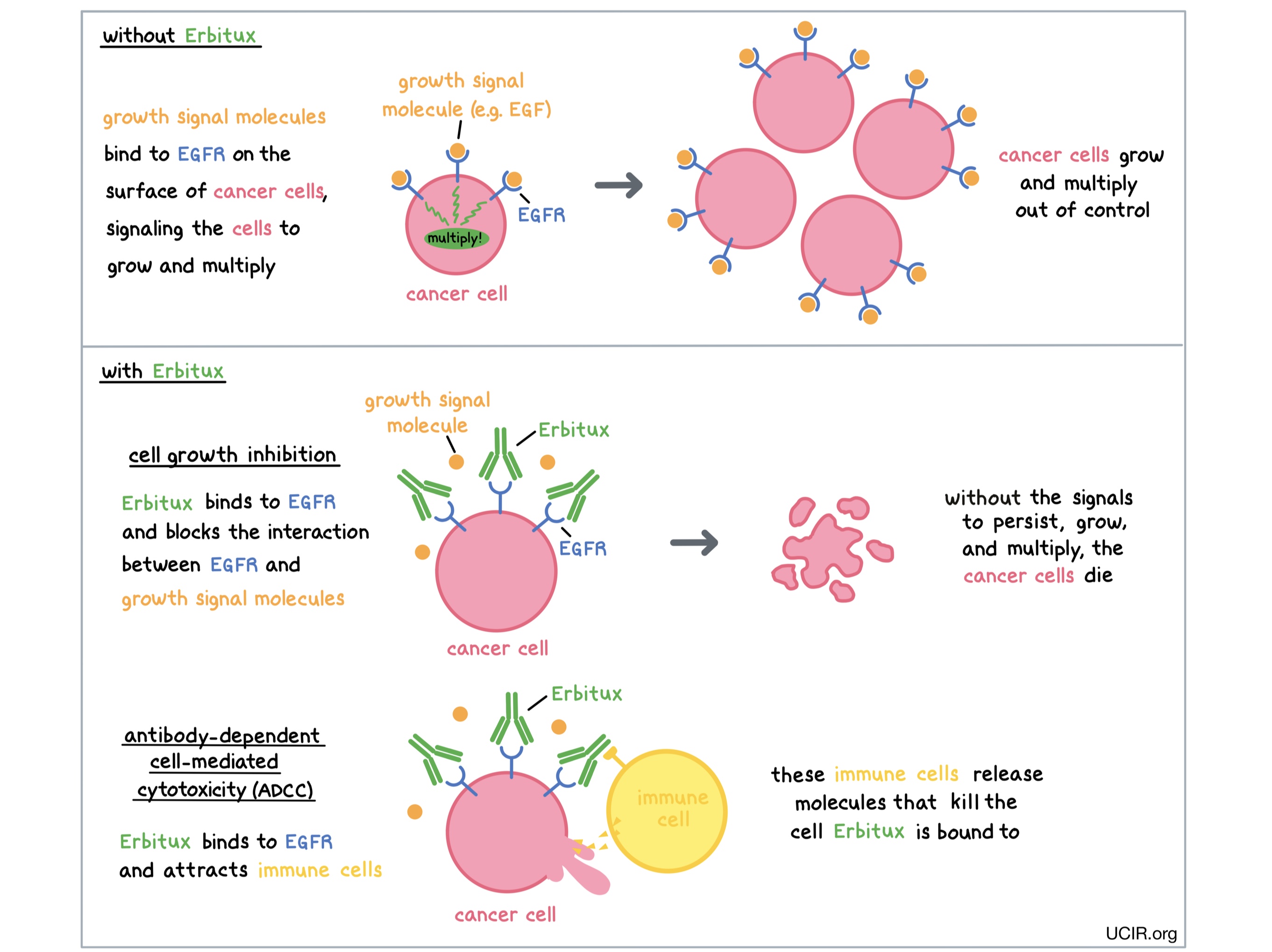
Erbitux is an antibody that was made in the laboratory and is designed to attach to a protein molecule called epidermal growth factor receptor (EGFR). EGFR is present on the surface of many normal cells (such as cells in the skin and hair follicles), and it is also present in much higher quantities on the surface of many types of cancer cells, including those of the head and neck, colon, and rectum. Higher-than-normal amounts of EGFR on cancer cells make these cells the main target of Erbitux.
Erbitux and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. The stem of Erbitux’s “Y” shape can attract immune cells or other parts of the immune system.
Erbitux works to kill cancer cells in at least two ways.
Cancer cell growth inhibition When certain molecules (such as epidermal growth factor [EGF]) bind to EGFR on the surface of cells, the cells receive signals that ensure their survival and encourage them to grow and multiply. Higher-than-normal amounts of EGFR allow cancer cells to grow and multiply out of control. By binding to EGFR, Erbitux blocks these signals and prevents the cells from persisting and multiplying.
Antibody-dependent cell-mediated cytotoxicity (ADCC) When bound to EGFR on the surface of cancer cells, the “stem” of Erbitux can attract and bind immune cells (like NK cells). This allows Erbitux to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell Erbitux is bound to.
Colorectal cancer cells that contain mutated RAS genes do not depend on EGFR for their survival and growth, and can multiply out of control even when EGFR is blocked.
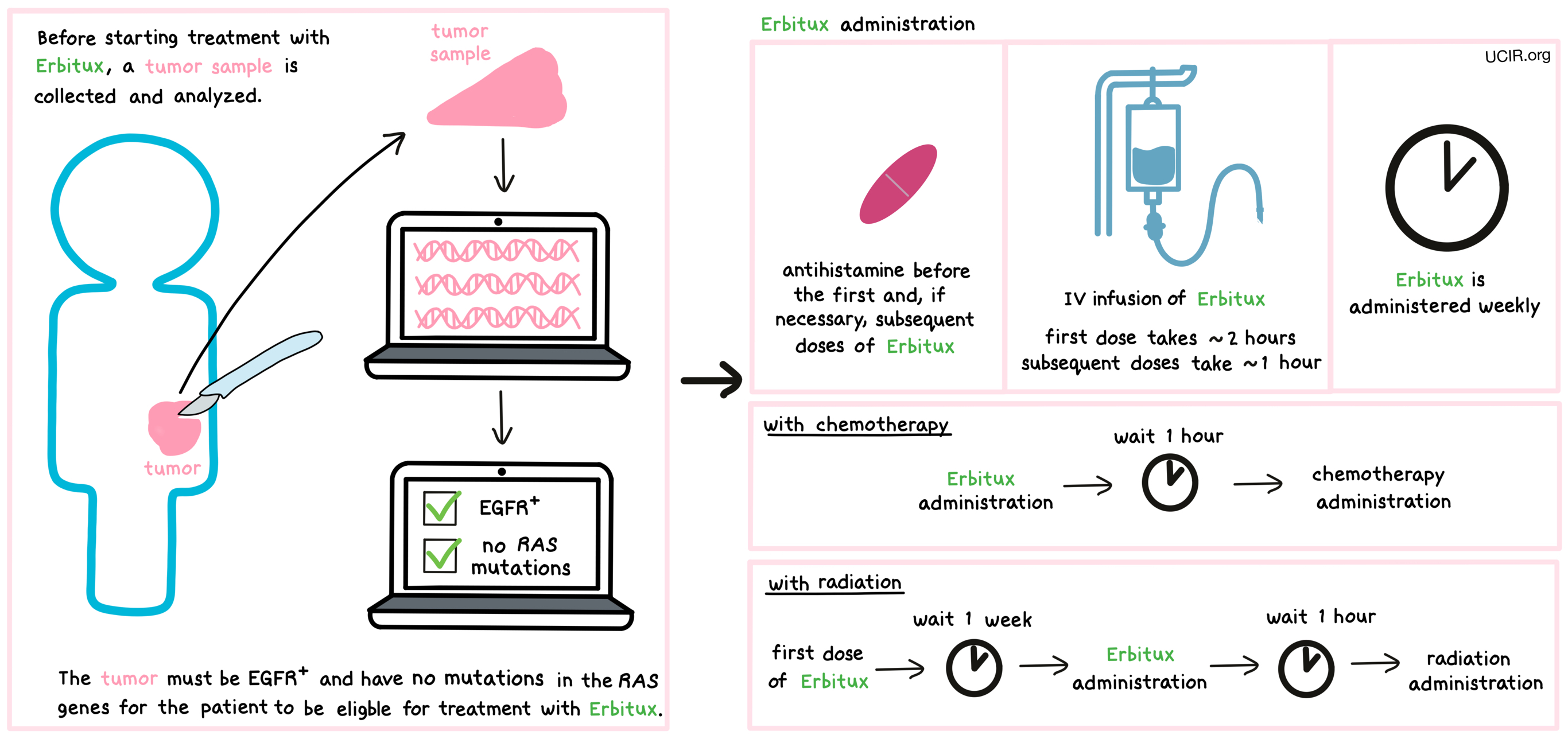
How is this drug given to the patient?
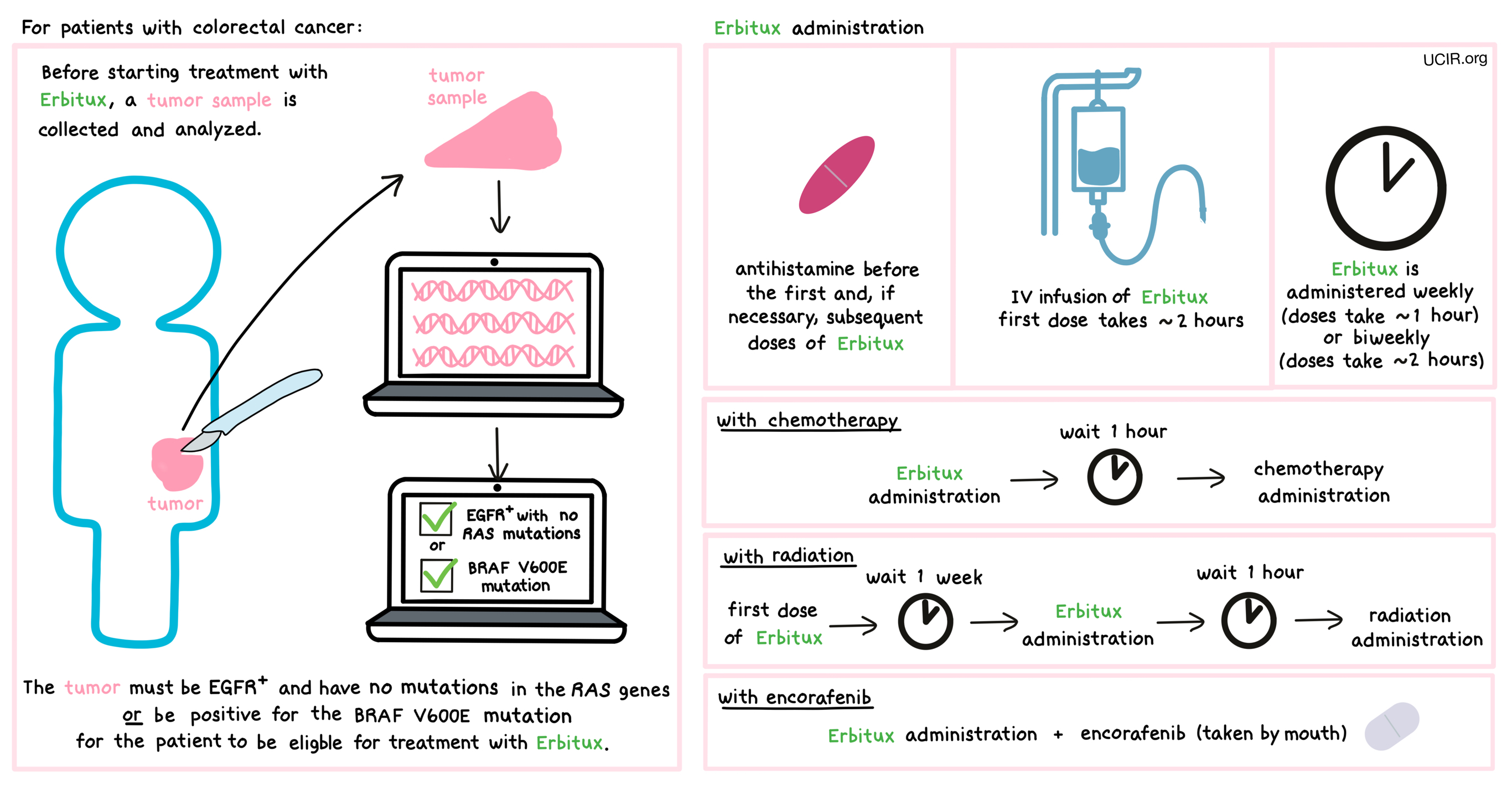
Before starting treatment with Erbitux, a small tumor sample from patients with colorectal cancer is collected and tested to determine:
- if the tumor is positive for the EGFR protein and does not have mutations in the RAS genes (including KRAS), OR
- if the tumor is positive for the BRAF V600E mutation.
Half an hour to an hour prior to administration of the first and, if necessary, subsequent doses of Erbitux, patients receive an antihistamine (diphenhydramine) to help reduce the chance of a reaction to the infusion.
Erbitux is administered via a tube into a vein (intravenous infusion, or i.v.) over 2 hours. Subsequent doses are given weekly over 1 hour or biweekly over 2 hours. When used in combination with radiation or chemotherapy, Erbitux administration should be completed 1 hour prior to radiation or chemotherapy. When used in combination with radiation therapy, the first dose of Erbitux is administered 1 week prior to radiation therapy.
What are the observed clinical results?
For:
Head and neck squamous cell cancer (treated with Erbitux and radiation therapy)
Head and neck squamous cell cancer (treated with Erbitux and chemotherapy)
Head and neck squamous cell cancer (treated with Erbitux alone)
Colorectal cancer (treated with Erbitux and combination chemotherapy)
Colorectal cancer (treated with Erbitux and irinotecan chemotherapy)
Colorectal cancer (treated with Erbitux alone)
Colorectal cancer (treated with Erbitux and encorafenib)
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Head and neck squamous cell cancer (treated with Erbitux and radiation therapy)
In a clinical trial, 424 patients with previously untreated head and neck squamous cell cancer that had spread to nearby tissue or lymph nodes, were treated with either Erbitux plus radiation therapy, or radiation therapy alone.
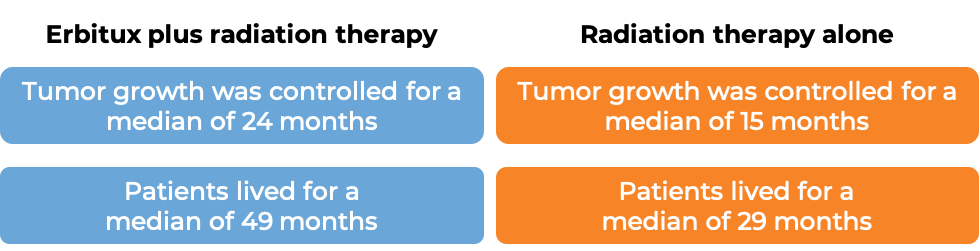
(For the definition of “median”, click HERE.)
Head and neck squamous cell cancer (treated with Erbitux and chemotherapy)
In a clinical trial, 442 patients with head and neck squamous cell cancer that had spread to nearby tissue or lymph nodes or other parts of the body, and who had not received treatment for their advanced disease, were treated with Erbitux plus platinum-based chemotherapy and fluorouracil, or platinum-based chemotherapy and fluorouracil alone. At a median follow-up of 18 to 19 months:
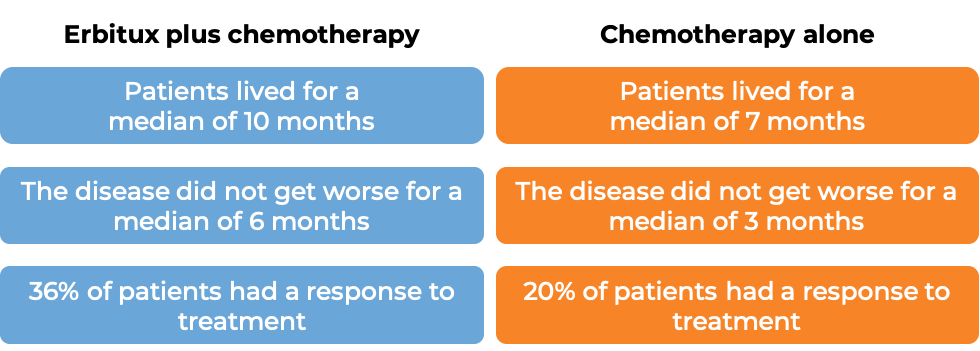
Head and neck squamous cell cancer (treated with Erbitux alone)
In a clinical trial, 103 patients with previously treated head and neck squamous cell cancer that had grown or spread to other parts of the body, who had received treatment with platinum-based chemotherapy and the disease kept getting worse, were treated with Erbitux alone. 13% of patients had a response to treatment and responses lasted for a median of 6 months.
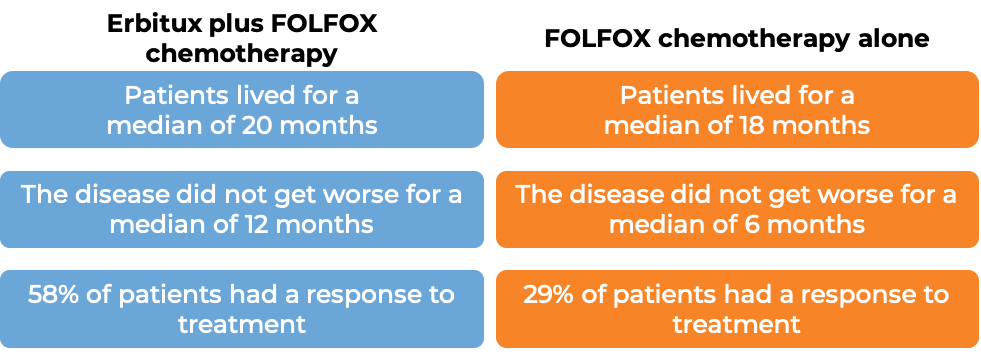
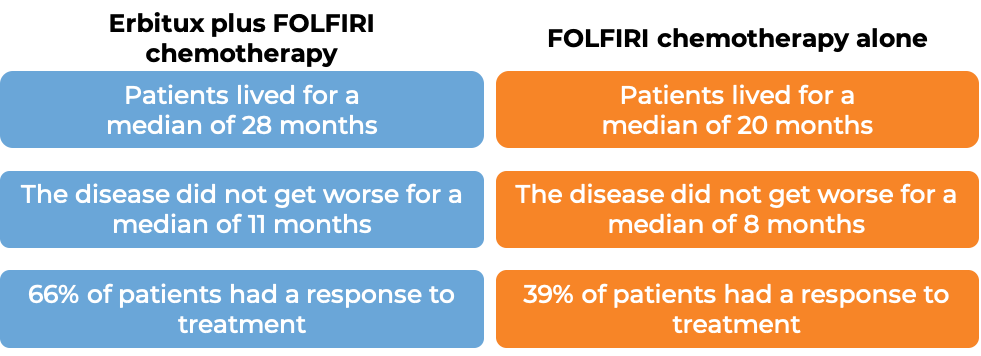
Colorectal cancer (treated with Erbitux and combination chemotherapy)
In a clinical trial, 1217 patients with advanced colorectal cancer that had spread to other parts of the body, were treated with either Erbitux plus irinotecan, fluorouracil, and leucovorin chemotherapy, or irinotecan, fluorouracil, and leucovorin chemotherapy alone. Among the 676 patients whose cancer tested positive for the EGFR molecule and had normal (“wild-type”) copies of the KRAS gene, at a median follow-up of 29 to 30 months:
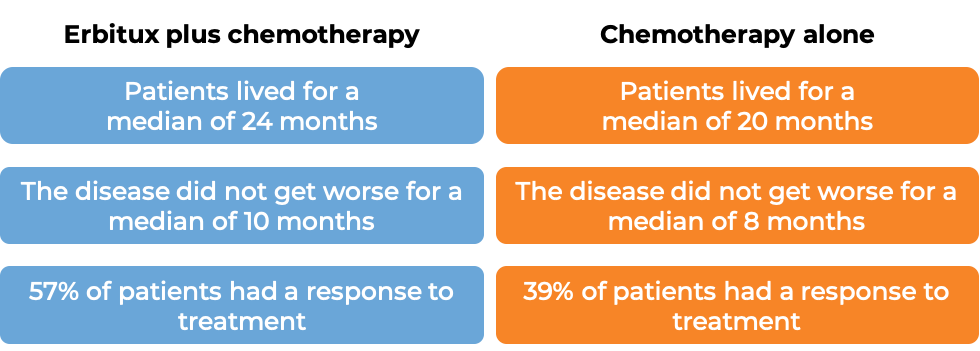
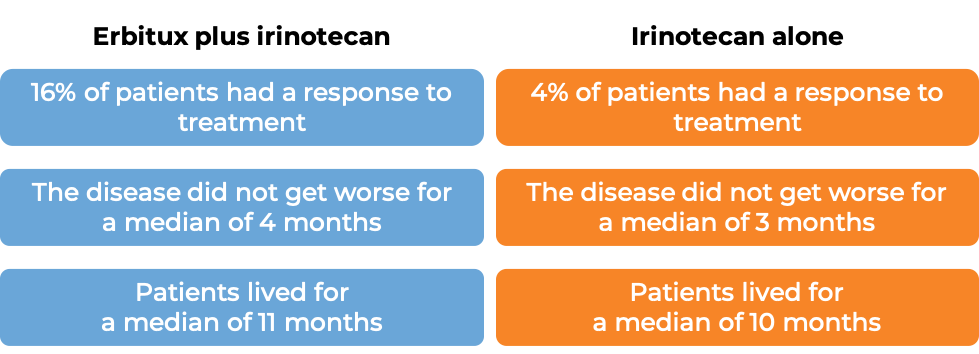
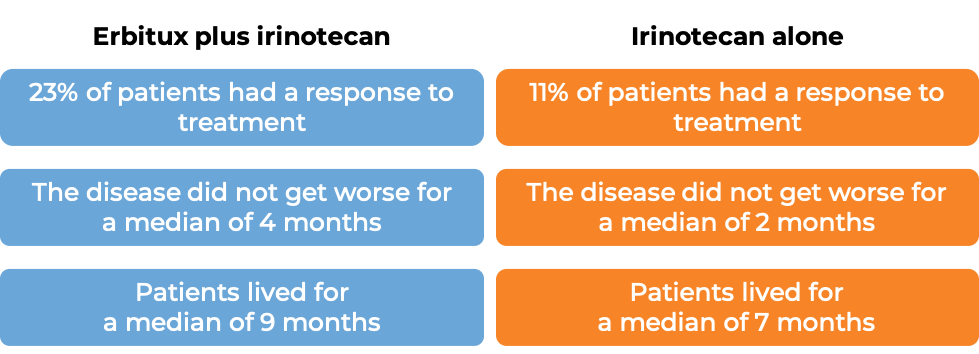
Colorectal cancer (treated with Erbitux and irinotecan chemotherapy)
In a clinical trial, 329 patients with advanced colorectal cancer that had spread to other parts of the body, were treated with either Erbitux plus irinotecan, or Erbitux alone. Tumors were positive for the EGFR molecule but not tested for mutations in the KRAS gene.
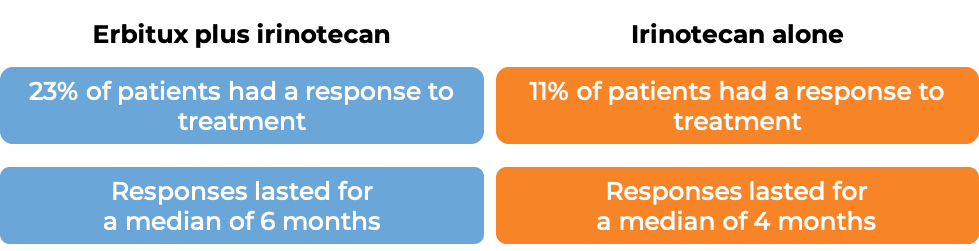
(For the definition of “median”, click HERE.)
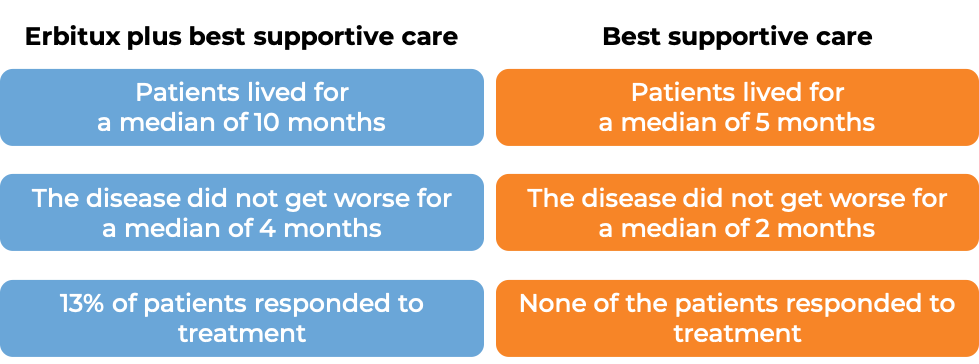
Colorectal cancer (treated with Erbitux alone)
In a clinical trial, 572 patients with advanced colorectal cancer that had spread to other parts of the body, who had been previously treated and the treatment did not work or stopped working, were treated with either Erbitux plus best supportive care, or best supportive care alone. Among the 245 patients whose cancer tested positive for the EGFR molecule and had normal (“wild-type”) copies of the KRAS gene:
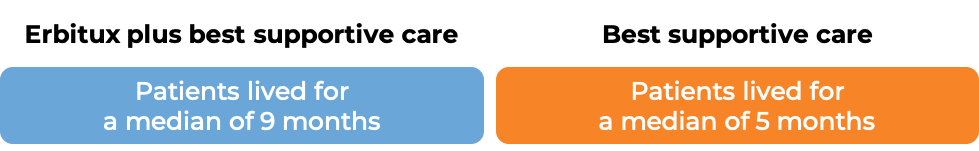
(For the definition of “median”, click HERE.)
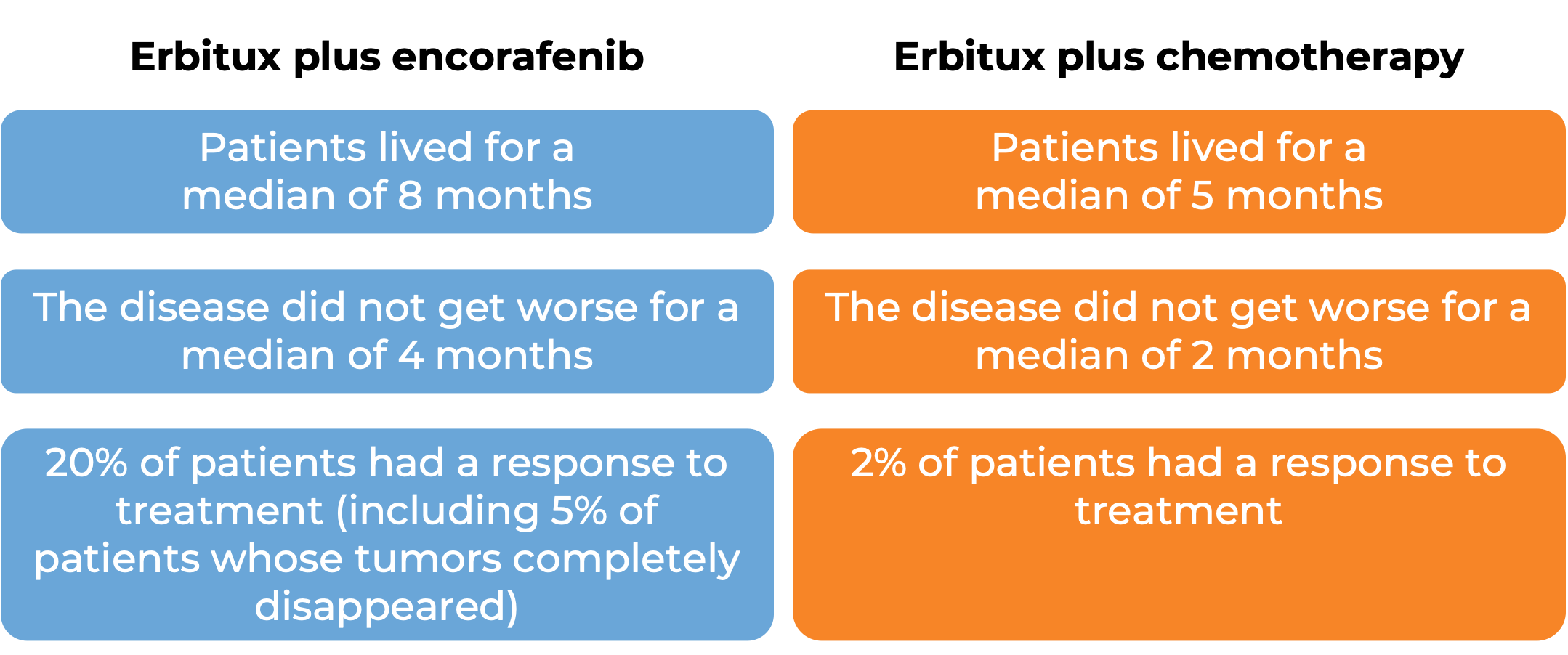
Colorectal cancer (treated with Erbitux and encorafenib)
In a clinical trial, 441 patients with previously treated colorectal cancer that had spread to other parts of the body and tested positive for the BRAF V600E mutation, were treated with either
- Erbitux and encorafenib, OR
- Erbitux and irinotecan or FOLFIRI (irinotecan, fluorouracil, and leucovorin) chemotherapy
At a median follow-up of 8 months:
What are the side effects?
The most common side effects of Erbitux include rash, itching, nail changes, headache, diarrhea, decreased appetite, and infection.
Erbitux can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Erbitux include severe skin reactions (which could lead to severe infections), severely low levels of electrolytes (including magnesium) in the body, problems with the heart (including heart attack), problems with the lungs (including interstitial lung disease, a scarring and thickening of tissue in the lungs that makes breathing difficult), and severe reactions related to the Erbitux infusion.
Infusion-related reactions Serious and life-threatening adverse reactions to the infusion of Erbitux have been observed. Infusion reactions may occur during or several hours following the infusion. Patients should be monitored for at least 1 hour following each Erbitux infusion. Symptoms of an infusion-related reaction include hives or rash, itchiness, swelling of the tongue, lips, face, or throat, coughing, shortness of breath, wheezing, difficulty breathing, weakness, dizziness, shock, feeling as if your heart is fluttering or racing, and chest pain. The risk of severe allergic reactions to the infusion may be increased in patients with a history of tick bites, red meat allergy, or if they have a particular type of antibody (antibodies directed against alpha-gal) in their blood.
Low levels of magnesium in the body (hypomagnesemia) Symptoms of low levels of magnesium in the body (hypomagnesemia) include involuntary twitching, especially of the facial muscles, physical weakness or an unexplained feeling of exhaustion, tremors, constipation, nausea or vomiting, muscle cramps, personality changes, fluttering sensations in the chest, or chest pain. If left unaddressed, hypomagnesemia can lead to severe heart problems, such as prolonged uneven heartbeats. Low levels of magnesium or other electrolytes (including calcium and potassium) can occur days to months after beginning treatment with Erbitux. Patients are monitored weekly during treatment, and for at last 8 weeks after completion of Erbitux treatment, for low electrolyte levels in their body.
Skin reactions Erbitux can cause severe and life-threatening skin reactions, including infection at the sides of finger or toe nails, rash, skin inflammation that looks like acne, itching, red skin, dry skin, and blistering of mucus membranes in the mouth, nose, and around the eyes. Exposure to sunlight could worsen skin reactions, and patients are advised to limit sun exposure and wear sunscreen and hats.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Eli Lilly and Company (US)
Merck KGaA (Europe)
Approval
FDA and EMA
Links to drug websites
- US: https://www.erbitux.com/locally-advanced-scchn/what-is-erbitux
- Europe: https://www.ema.europa.eu/en/medicines/human/EPAR/erbitux
Other references
- Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. Vermorken JB et al. The New England Journal of Medicine (2008)
- Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. Van Cutsem E et al. The New England Journal of Medicine (2009)
- Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. Kopetz S et al. The New England Journal of Medicine (2019)
Last updated on March 11, 2022