Antibody Therapy
What is antibody cancer immunotherapy?
Antibodies are a type of molecule that the human immune system uses to detect and target things that don’t belong in the body, including bacteria, viruses, and cancer cells. The targets of antibodies can be molecules floating freely in body fluids, molecules on the surface of invading viruses or bacteria, or molecules on the surface of a cell in the body. Antibodies can also very precisely recognize molecules that differentiate cancer cells from normal cells. By binding to the cancer cells, antibodies may work alone or may help the immune system to fight the cancer.
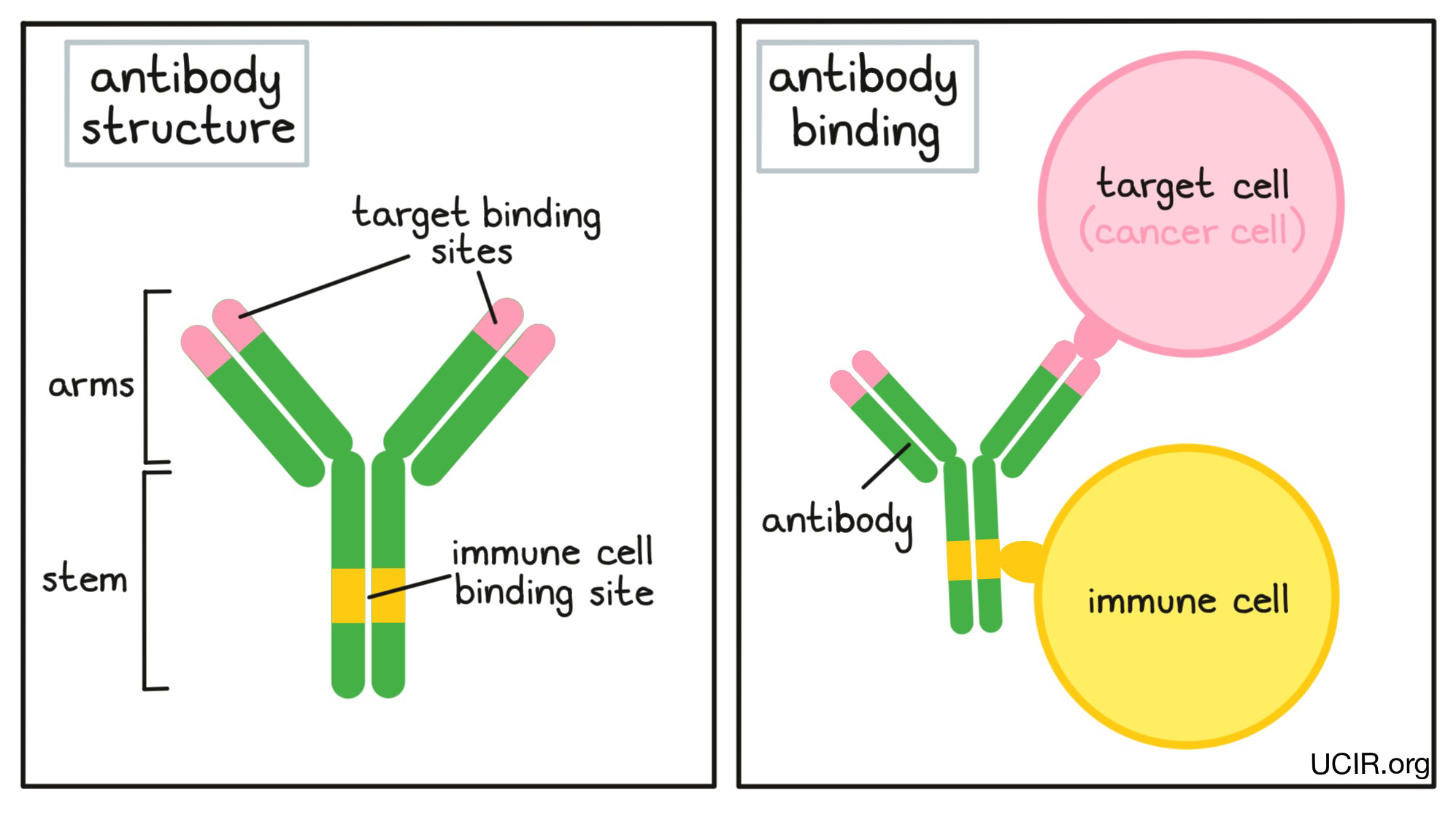
An antibody molecule has an overall “Y” shape. The tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to target molecules, fitting onto them like puzzle pieces. Because the two tips of the “Y” arms on an antibody are identical, one antibody can potentially bind two targets at the same time. The stem of the “Y” of the antibody has an immune cell binding site, which allows the antibody to act as a bridge between the target cell and the immune cell. For example, if a target cell is bound to the arm of an antibody, and an immune cell binds to the stem, then the immune cell can attack and destroy the target cell.
Antibodies are produced by immune cells called B cells. There are as many as a billion different B cells in a person’s body that circulate in search of invading organisms or molecules that don’t belong. Each B cell produces its own unique antibody, which allows the immune system to be prepared against almost any imaginable threat. When a B cell finds a target that its antibody can bind to, it matures and multiplies. This results in an army of identical B cells that can all produce and release the same antibody.
Cancer arises when normal cells in the body mutate and begin to multiply out of control. Since cancer cells are mutated, they may produce molecules that are abnormal enough to be recognized by the immune system, marking them as cells that don’t belong. When this happens, the immune system will react to the cancer cells in the same way it reacts to bacteria or viruses, and B cells will begin to produce antibodies, which may serve a number of different functions. Antibodies may block the function of abnormal molecules on cancer cells, stop or slow down tumor growth, stop cancer cells from spreading to other parts of the body, mark cancer cells for elimination by the immune system, or amplify an immune response. If the body’s natural immune response to the cancer is not strong enough, however, the cancer will continue to grow.
While antibodies are produced naturally by the body, scientists have found ways to develop and manufacture antibodies in laboratories for use as cancer-fighting drugs. When scientists want to utilize a particular antibody, they identify the B cells that make it, grow the B cells, and harvest the antibodies. Further, scientists can create modified antibodies, either by altering the genetic code of the B cells so that they produce antibodies with more desirable properties, or by changing the antibodies after they have been produced.
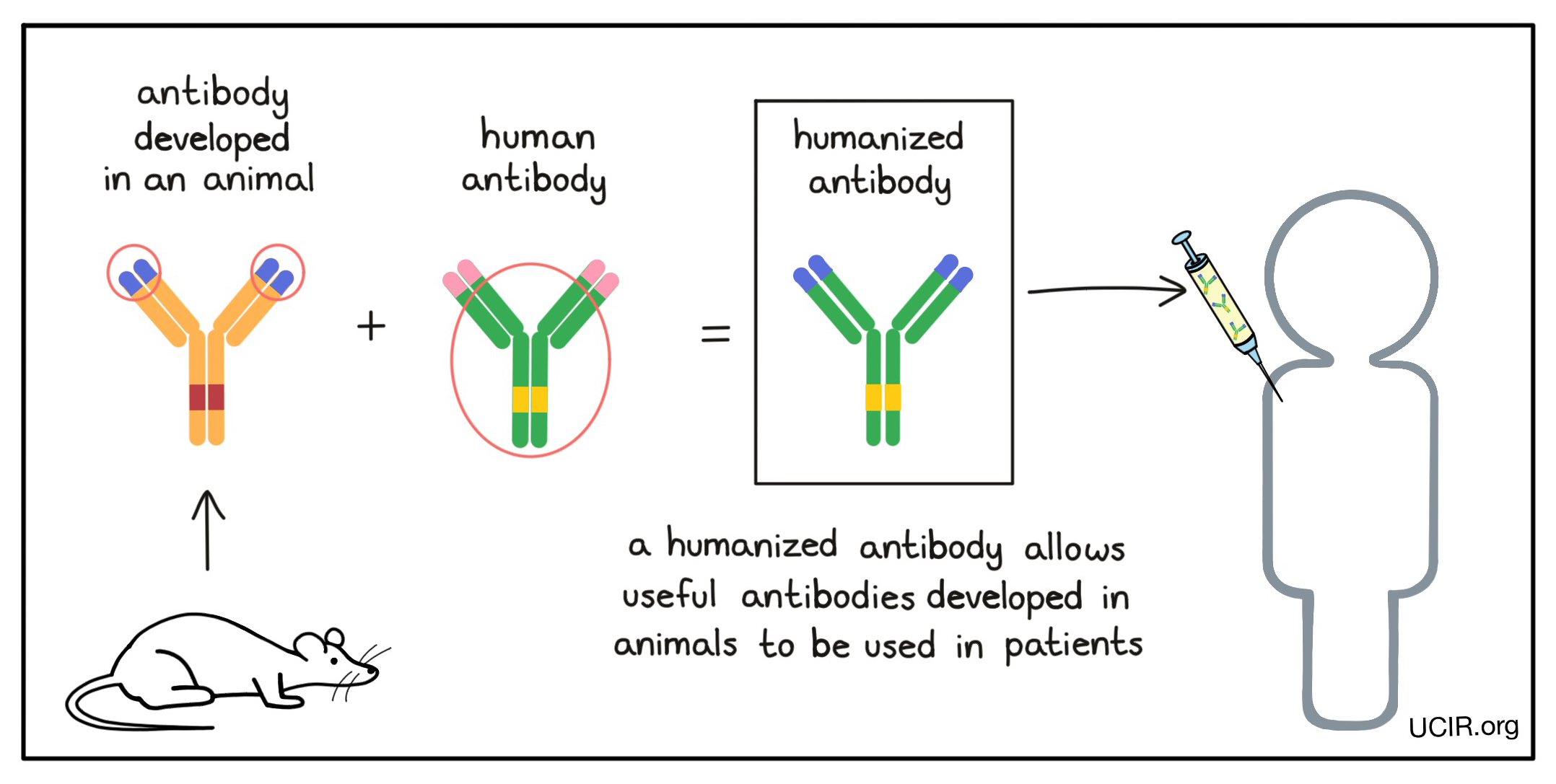
Many useful antibodies are developed in mice (or other animals), but before they can be used in patients, these antibodies need to be “humanized” to prevent them from being recognized as “foreign” by the patient's immune system. Humanized antibodies are made by changing the genetic code of an antibody that was developed in an animal and replacing all but the target binding sites (the tips of the arms of the “Y”) with the genetic code from a human antibody. Some antibody drugs may also be labeled as “chimeric” antibodies; this means that they are nearly humanized, but a little more of the original animal antibody was kept.
Strategies for using antibodies in cancer immunotherapy
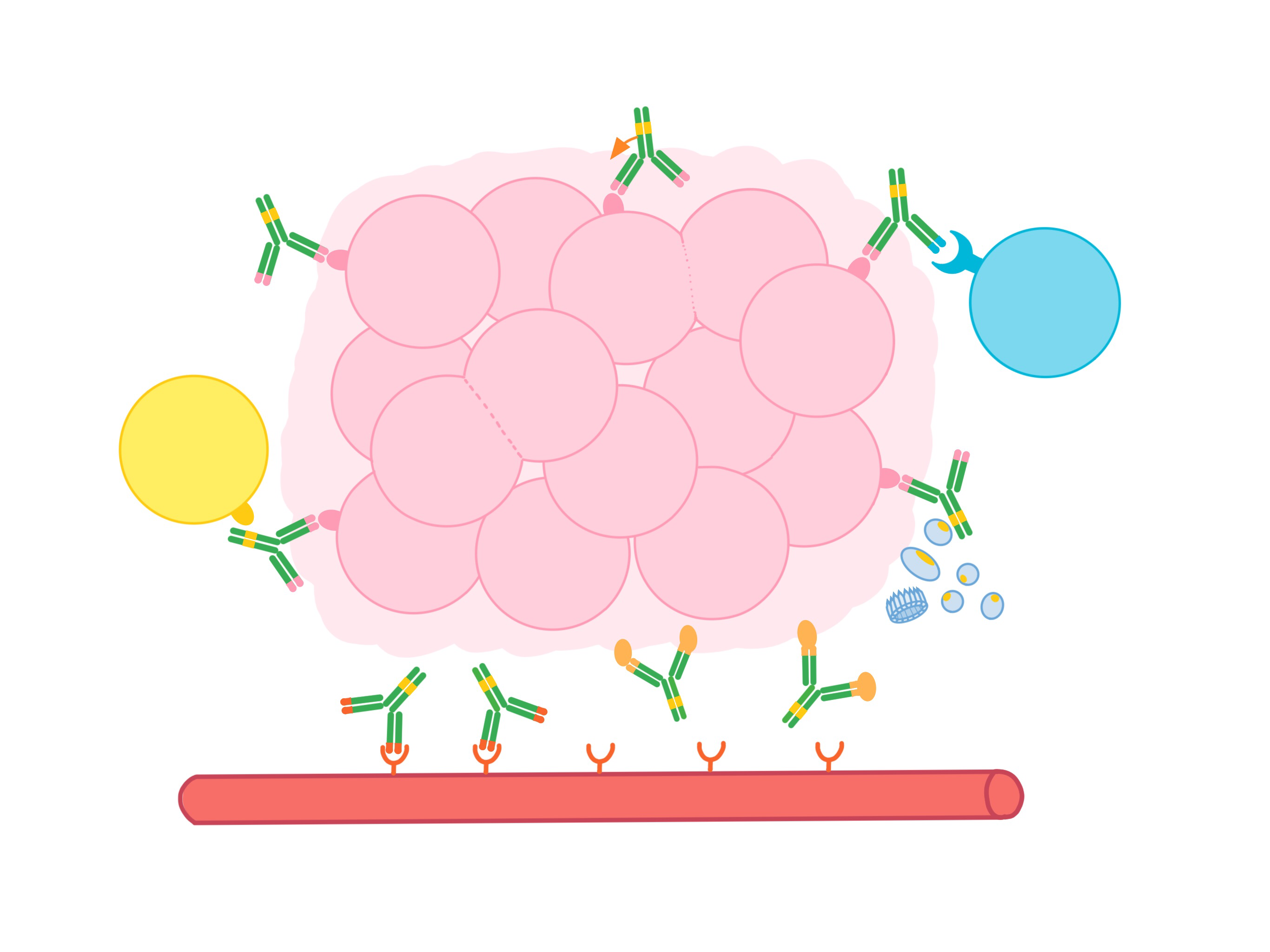
Antibodies are used in cancer immunotherapy to:
- Block the activity of molecules that encourage the cancer to multiply or spread to other parts of the body (metastasize)
- Block the activity of cells that help nourish tumors
- Deliver cell-killing molecules to cancer cells
- Recruit other parts of the immune system to kill cancer cells
- Link immune cells to cancer cells to boost tumor killing
- Keep antitumor immune cells from shutting down
For a more complete list of currently approved antibody therapies, click here.
Antibodies can block the activity of molecules that encourage the cancer to multiply or spread to other parts of the body (metastasize)
Cancer cells have many different molecules on their surfaces that act like sensors, telling the cells what’s going on in their environment. When these sensors are triggered by external signals, they may tell cancer cells to do things like multiply or migrate. Antibodies can be used to block these sensors (or the signal molecules that bind to the sensors), preventing the sensors from telling the cancer cells what to do.
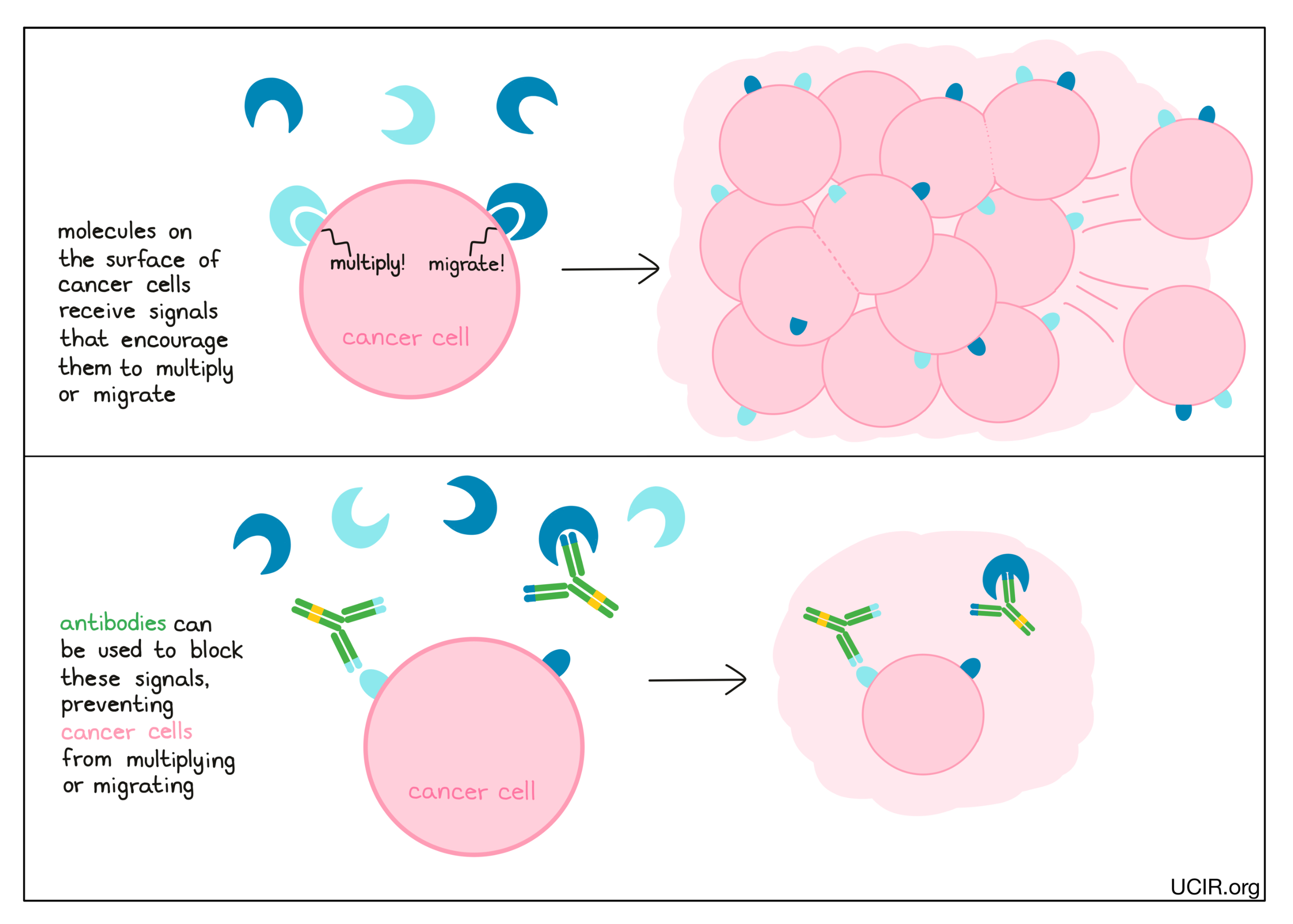
Examples:
Anti-HER2
HER2 (Human Epidermal growth factor Receptor 2) is a molecule found on the surface of normal cells. When one molecule of HER2 binds to another molecule of either HER2 or a molecule that closely resembles HER2 (like HER3) on the surface of the same cell, the pair sends a signal to the cell that it is time to multiply. In cancer cells, the number of HER2 molecules may be significantly increased, leading to more pairings of HER2 molecules and thus, more multiplication of the cancer cells. Antibodies have been developed to bind to HER2 on the surface of cancer cells and prevent HER2 from forming signaling pairs. In addition, antibodies bound to HER2 can lead to HER2 molecules being removed from the cell’s surface, thus further reducing growth signaling. One prominent anti-HER2 antibody is called trastuzumab.
Anti-IL-6
IL-6 (Interleukin 6) is a molecule that circulates throughout the body and can bind to a variety of cells, stimulating them in different ways. Recent studies have shown that IL-6 may help cancer cells to spread and “seed”, forming tumors in new locations. Based on these findings, scientists developed an antibody called siltuximab, which blocks the binding of IL-6 to its receptor on the surface of cells. Siltuximab was approved for the treatment of Multicentric Castleman's Disease, a cancer-like disease that depends on IL-6 signaling for growth.
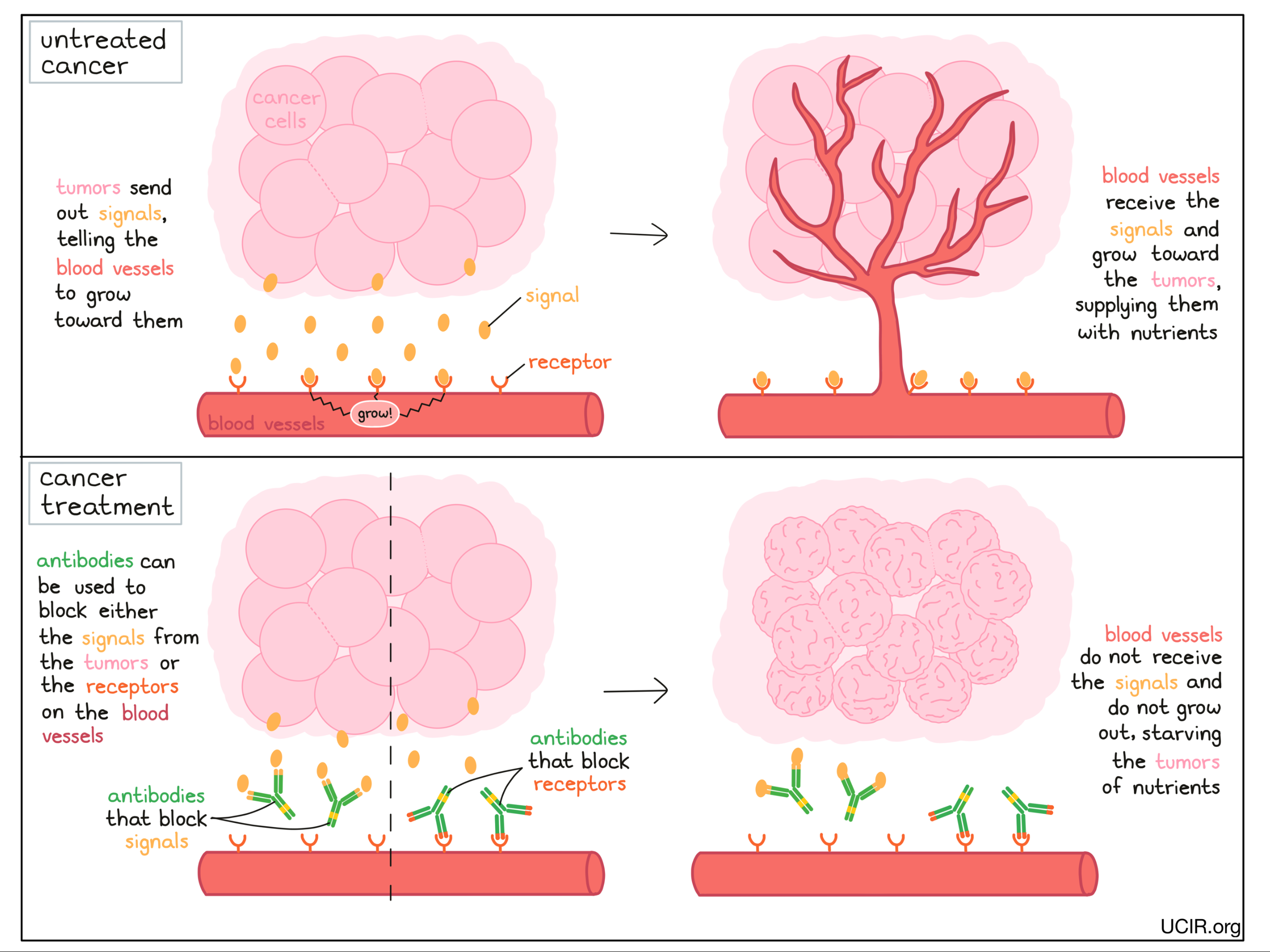
Antibodies can block the activity of cells that help nourish tumors
In the same way that antibodies can be used to block tumor growth, they can also be used to block the growth of blood vessels that support the growing tumors. Tumors send signal molecules that essentially ask the blood vessels to grow towards them. Antibodies can be used to block those signals, or the receptors on the blood vessels that receive these signals. This prevents blood vessels from growing toward the tumors, starving the tumor of nutrients and preventing the tumor from continuing to grow.
Examples:
Anti-VEGF and anti-VEGFR
Tumors require a blood supply to sustain their growth. VEGF (Vascular Endothelial Growth Factor) is a molecule that helps to stimulate the formation of new blood vessels. To prevent the formation of new blood vessels in tumors, scientists developed an antibody called bevacizumab, which binds to VEGF and prevents it from signaling for blood vessel development. Without blood vessel development, tumors are starved of nutrients and cannot continue to grow.
An alternative antibody called ramucirumab also blocks the ability of VEGF to stimulate blood vessel growth by binding to and blocking the VEGF receptor on the blood vessels, thus preventing blood vessel development and tumor growth.
Antibodies can deliver cell-killing molecules to cancer cells
Since antibodies bind very specifically to their intended targets, they can be used to deliver chemotherapies or radioactive molecules directly to cancer cells, avoiding delivery to normal cells. Because the stem end of the “Y” shaped antibody molecule is not necessary for binding to a target, scientists can use this end of the molecule as a site to attach radioactive or cell-killing molecules. Within patients, the antibodies seek out and bind to cancer cells. The cancer cells then take in the antibodies, which deliver and release their deadly payload. Finding a target that is unique to the cancer cells or is abundant on cancer cells, but not on normal cells, is a challenge. Once such a target is identified, this strategy can be implemented.
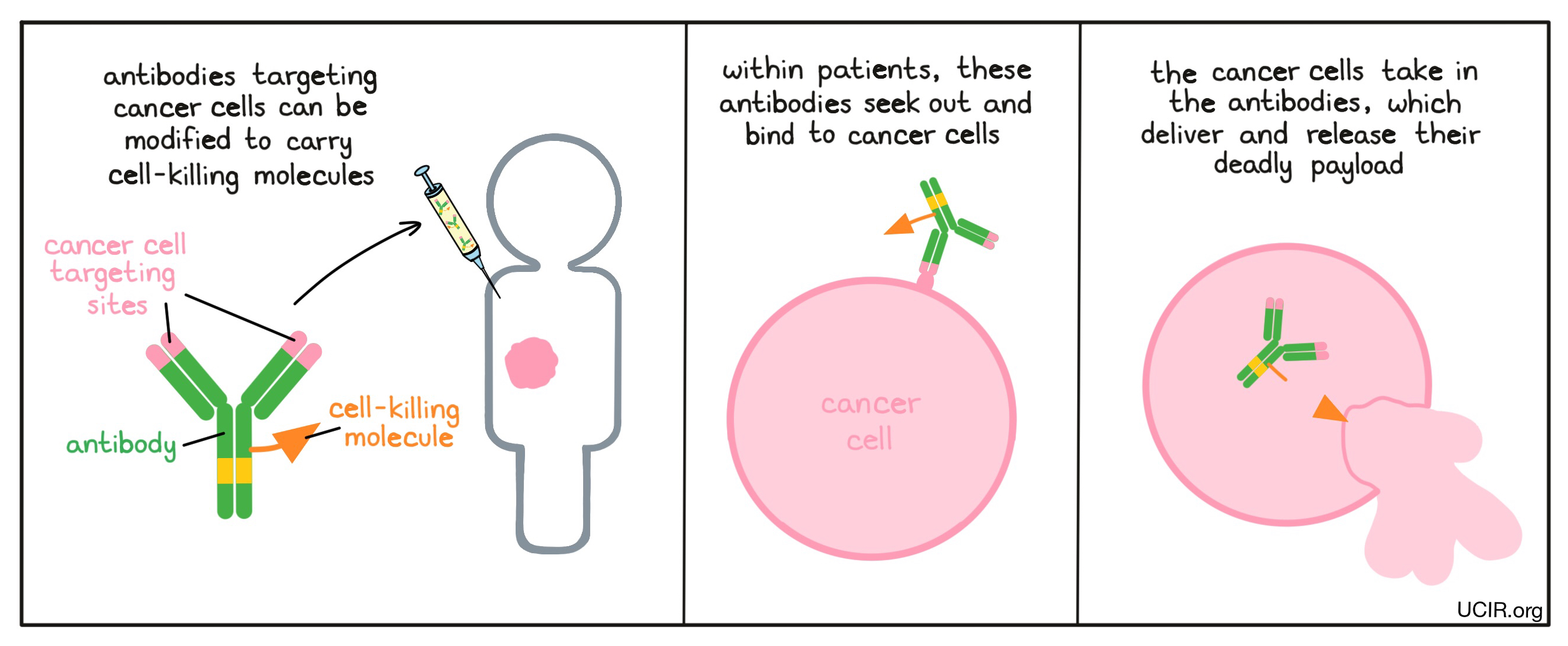
Examples:
Anti-CD30-MMAE
CD30 is a molecule that is present on the surface of some blood cell cancers (including anaplastic large cell lymphoma and Hodgkin lymphoma). Brentuximab vedotin is an antibody that binds to CD30 and delivers the cell-killing poison MMAE (monomethyl auristatin E) to cancer cells. MMAE can also leak out of the cancer cells and kill neighboring cancer cells. The dying cancer cells and the cancer cell debris can further alert the immune system and enhance an immune attack on the cancer cells.
Anti-HER2-Emtansine
The anti-HER2 antibody trastuzumab works by blocking the pairing of HER2 molecules, hindering them from passing on signals to the cancer cells, which would stimulate tumor growth (see above). Researchers have developed a version of the anti-HER2 antibody that can deliver the cell-killing molecule emtansine to cancer cells. The modified antibody, called trastuzumab emtansine, is used in patients with late-stage breast cancers that present high levels of HER2 on the surface of the cancer cells. When trastuzumab emtansine binds to HER2 it is taken into the cancer cell, where the emtansine payload can cause the cancer cell to die.
Antibodies can recruit other parts of the immune system to kill cancer cells
When antibodies bind to a target, the stems of the antibodies can kickstart other immune functions. The antibody stems contain binding sites for molecules of the immune system that freely flow in the blood or tissues, and molecules on the surface of certain immune cells. In a process known as Complement-Dependent Cytotoxicity (CDC), the stem ends of the antibodies activate molecules called “complement”, which can puncture the cells targeted by the antibodies. In another process known as Antibody-Dependent Cell-mediated Cytotoxicity (ADCC), the stem ends of antibodies attract nearby immune cells, such as NK cells or macrophages, which attack and kill the cells targeted by the antibodies. Changes to the stem of the antibody can be made to improve the ability of the antibody to attract and bind molecules of the complement system or immune cells.
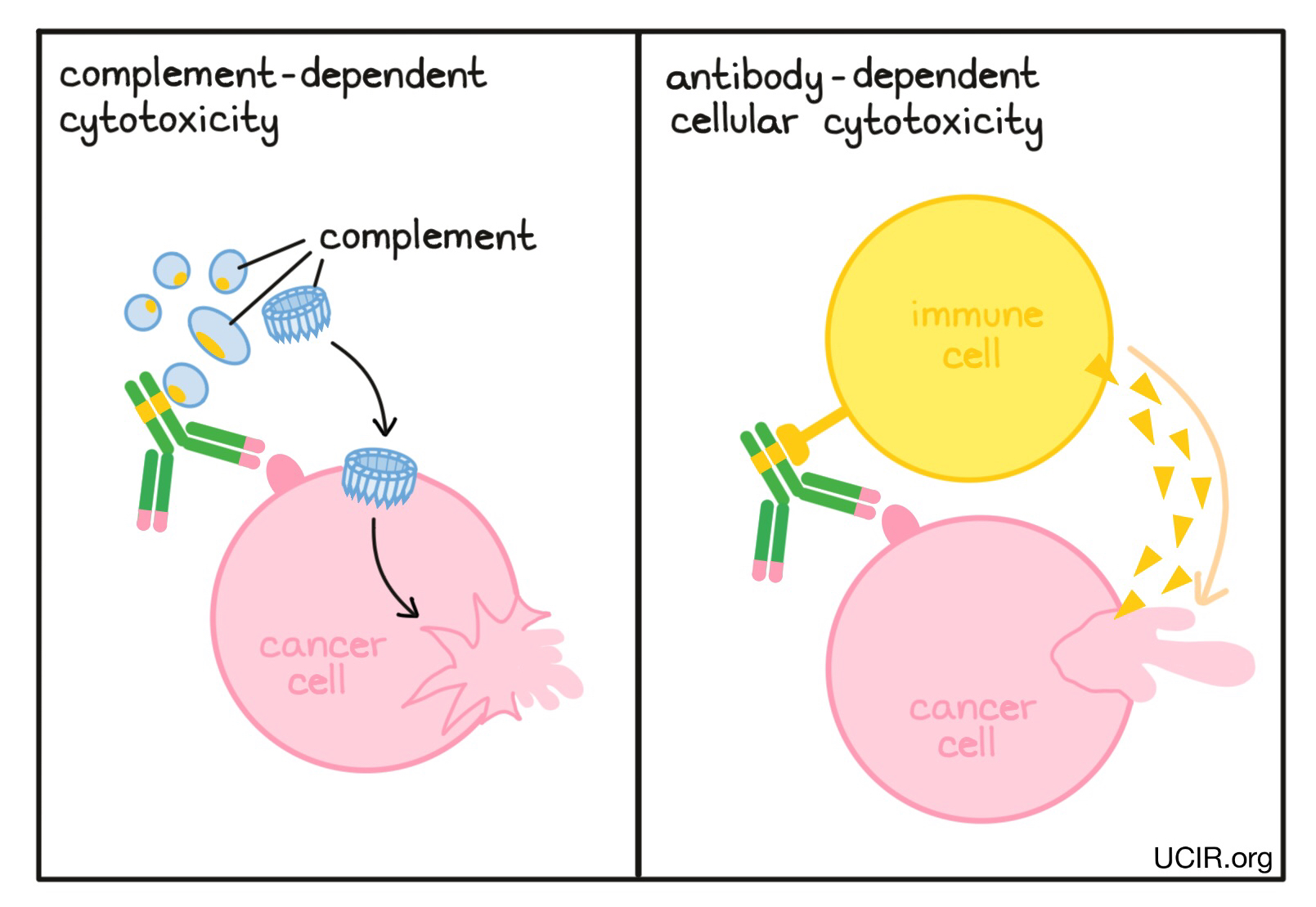
Example:
Anti-CD38
CD38 is a molecule found at low levels on the surface of many normal blood cells. However, multiple myeloma – a type of blood cancer – has particularly high levels of CD38. Daratizumab is an antibody that binds to CD38. The stem of daratizumab can attract and activate immune cells and molecules of the complement system to kill the cancer cells to which it is bound.
Antibodies can link immune cells to cancer cells to boost tumor killing
The two arms of an antibody can be modified so that the tip of each arm binds to different targets. Antibodies engineered with the ability to bind two different molecules are called bispecific antibodies and can be used to build a bridge connecting a cancer cell to an immune cell. One arm of the engineered antibody binds to a molecule that is unique to (or abundantly present on) cancer cells compared to normal cells, while the other arm binds to a molecule on the surface of a T cell (the key cell in the immune system’s fight against cancer) and turns on the cell-killing function of the T cell. In this way, the antibody acts like a deadly matchmaker, linking cancer cells up with the immune cells that could kill them.
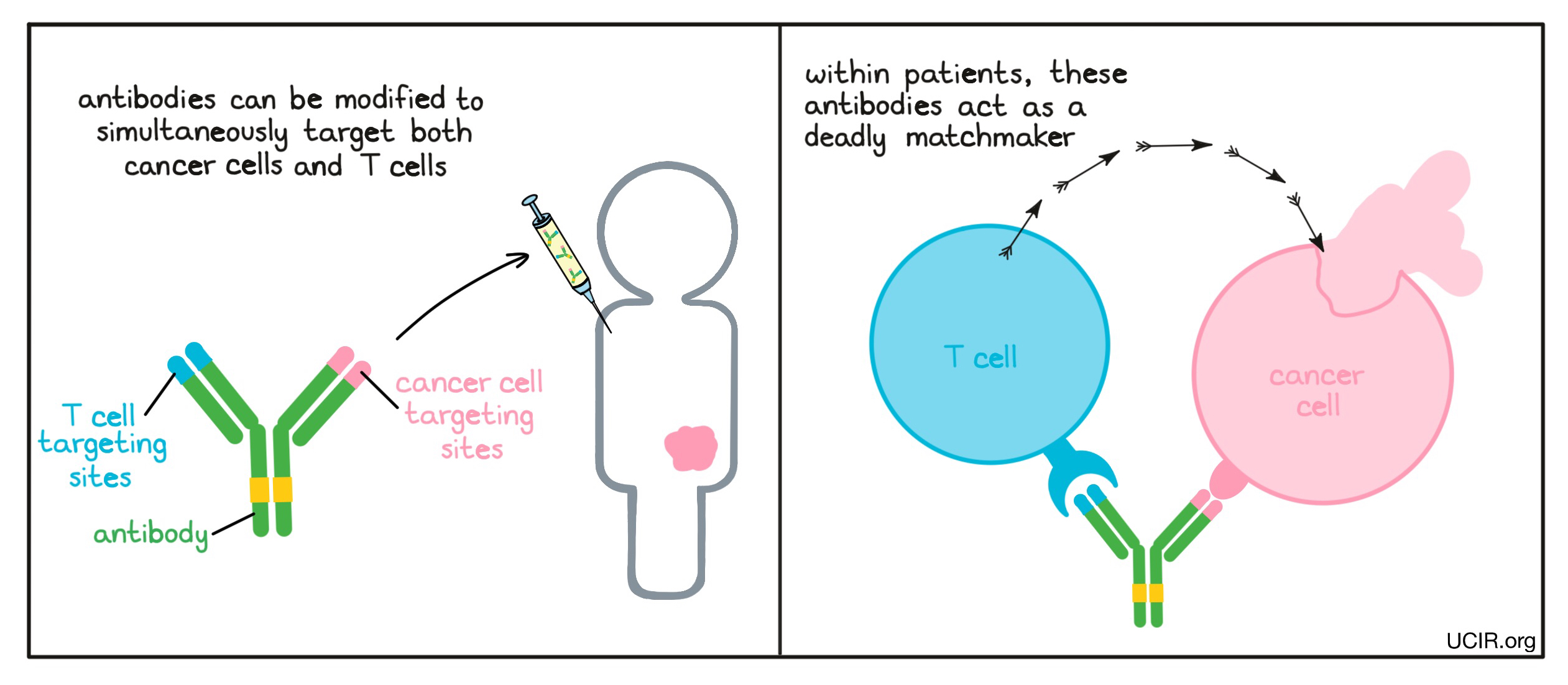
Example:
Anti-CD19-CD3 bridging molecule
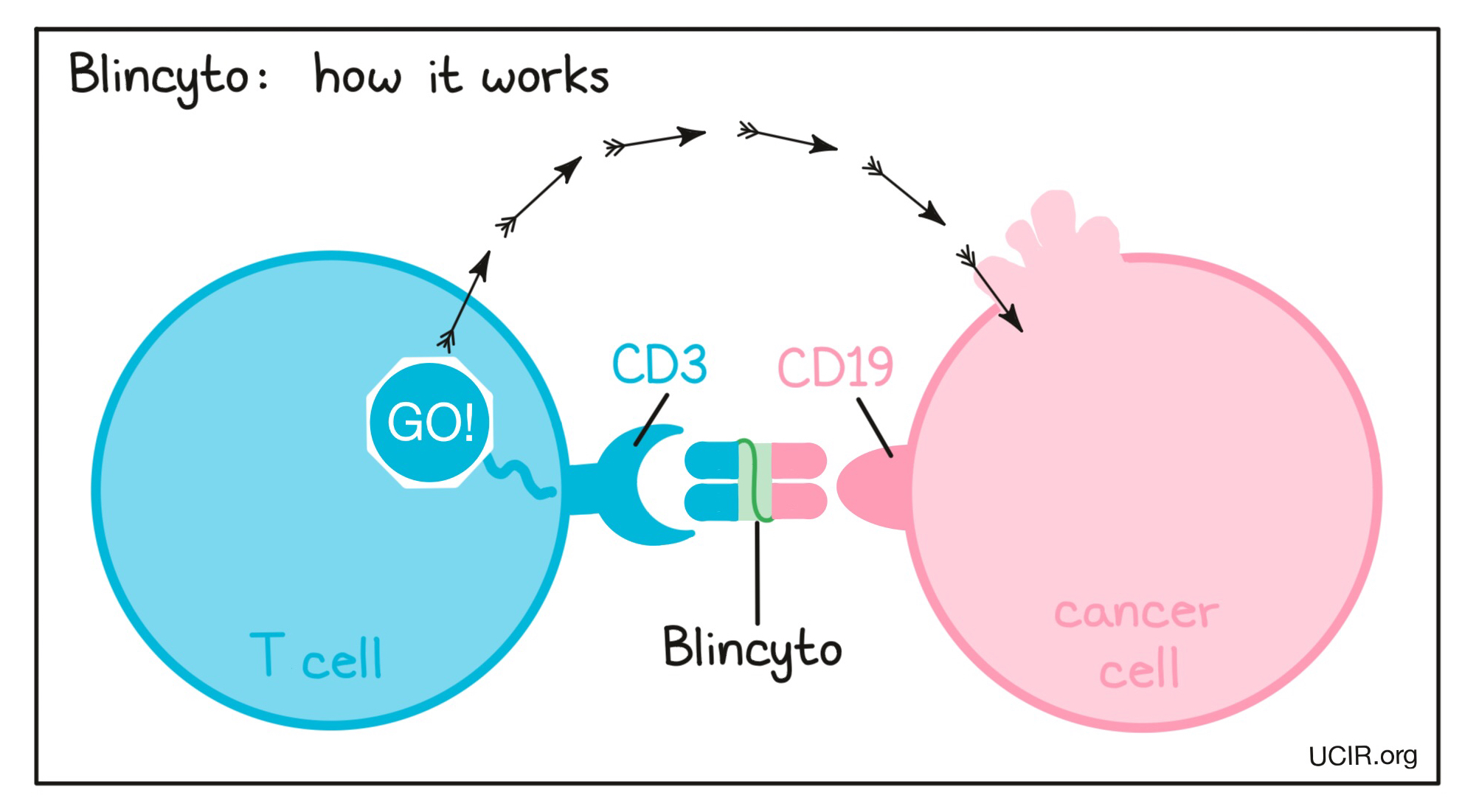
Blinatumomab, also called Blincyto, belongs to a class of engineered antibodies that link cancer cells with immune cells capable of killing their targets. One arm of Blincyto binds to CD19, a molecule found on the surface of most B cells but also on acute lymphoblastic leukemia (ALL) cells. The second arm binds to CD3, a protein found on T cells that is part of the T cell Receptor. The drug works by linking these two cell types. Binding CD3 on the T cell activates the T cell to kill the target cell it is linked to. Blincyto was the first approved bispecific antibody and is an example of how scientists can more dramatically engineer antibodies. Rather than just changing the tips of the arms of an antibody so that it could bridge two cells, scientists actually made a mini-antibody that consisted of only the two tips of the antibody arms.
Antibodies can keep antitumor immune cells from shutting down
The immune system is regulated by a complex network of signals, with many “checkpoints” in place to act as brakes and prevent excessive immune responses. While these checkpoints are critical for controlling normal immune responses, they can also shut down beneficial immune responses to cancer too soon. Antibody drugs can be used to block immune checkpoint molecules, essentially blocking the brakes of the immune cells and allowing for stronger immune responses against the cancer. Checkpoint blockade antibodies have become prominent in cancer immunotherapy and are commonly used in the clinic. For more detailed information, see checkpoint blockade therapy.
Examples:
Anti-CTLA-4
CTLA-4 is a molecule that appears on the surface of activated T cells – the key cells in the immune system’s fight against cancer. When CTLA-4 molecules are triggered, they send a “stop” signal to the T cell. An antibody drug called ipilimumab blocks CTLA-4 and prevents it from being triggered, which helps sustain T cell activity against cancer. Ipilimumab has been effective and is approved for the treatment of metastatic melanoma, renal cell carcinoma, and colorectal cancer.
Anti-PD-1
PD-1 is a molecule that appears on the surface of T cells after activation. When PD-1 molecules are triggered, they send a “stop” signal to the T cell. Several antibody drugs have been developed to block PD-1 and prevent the “stop” signal from being triggered, which helps sustain T cell activity against cancer. Further, some antibody drugs have been developed to block the molecule PD-L1, which is the molecule that triggers PD-1. The effect of blocking PD-L1 is very similar to the effect of blocking PD-1.