How is this drug name pronounced?
Brentuximab Vedotin: bren-TUK-sih-mab veh-DOH-tin
Adcetris: ad-SEH-tris
What cancer(s) does this drug treat?
Classical Hodgkin lymphoma
Adcetris is approved for:
- Adult patients with newly diagnosed, previously untreated Stage III or IV classical Hodgkin lymphoma. In such cases, Adcetris is used in combination with chemotherapy (doxorubicin, vinblastine, and dacarbazine).
- Adult and pediatric patients 2 years and older with previously untreated classical Hodgkin's lymphoma who are at high risk of their disease becoming worse. In such cases, Adcetris may be given in combination with doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide chemotherapy.
- Adult patients with classical Hodgkin lymphoma who have received a stem cell transplant, and are at a high risk of their disease coming back or becoming worse. In such cases, Adcetris is given to prevent the cancer from coming back (consolidation therapy).
- Adult patients with classical Hodgkin lymphoma who have either received a stem cell transplant and the cancer came back, OR who have received at least two combination chemotherapy treatments that failed, and stem cell transplant is not an option.
T cell lymphoma
Adcetris is approved for:
- Adult patients with newly diagnosed, previously untreated systemic anaplastic large cell lymphoma (sALCL) or other peripheral T cell lymphomas that test positive for the CD30 molecule (including angioimmunoblastic T cell lymphoma and peripheral T cell lymphomas not otherwise specified). In such cases, Adcetris is used in combination with chemotherapy (cyclophosphamide and doxorubicin) and prednisone.
- Adult patients with systemic anaplastic large cell lymphoma (sALCL) who have received at least one combination chemotherapy, and the cancer came back.
- Adult patients with primary cutaneous anaplastic large cell lymphoma (pcALCL) or mycosis fungoides (MF) that tests positive for the CD30 molecule, who have previously received systemic therapy (treatment that affects the entire body) for their disease.
Limitations of use:
Age: The safety and efficacy of Adcetris have been established in pediatric patients age 2 or older with previously untreated classical Hodgkin's lymphoma who are at high risk of their disease becoming worse in combination with doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide chemotherapy. The safety and efficacy of Adcetris in patients under 18 years of age have not been established for any other indications.
Exclusions: Adcetris should not be administered to patients with severe kidney disease or patients with moderate or severe liver disease.
Fertility/Pregnancy/Breastfeeding: Adcetris may impair fertility in men, and may cause damage to sperm that could result in genetic abnormalities. Men should use effective contraception during treatment with Adcetris and for at least 4 months after their last dose of Adcetris. Adcetris may also impair fertility in females during treatment. Adcetris can cause harm to a fetus, and is not recommended for use during pregnancy. Women should not start Adcetris treatment when pregnant, and are advised to use effective contraception to prevent pregnancy for at least 2 months after the last dose of Adcetris. The risks associated with Adcetris during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Adcetris.
Interactions with other drugs: Adcetris should not be taken with bleomycin due to the risk of possible serious side effects to the lungs. Patients who are treated with medications called strong CYP3A4 inhibitors (e.g., ketoconazole) during Adcetris treatment should be closely monitored for signs of adverse reactions.
What type of immunotherapy is this?
- Antibody-drug conjugate
How does this drug work?
- Target: CD30
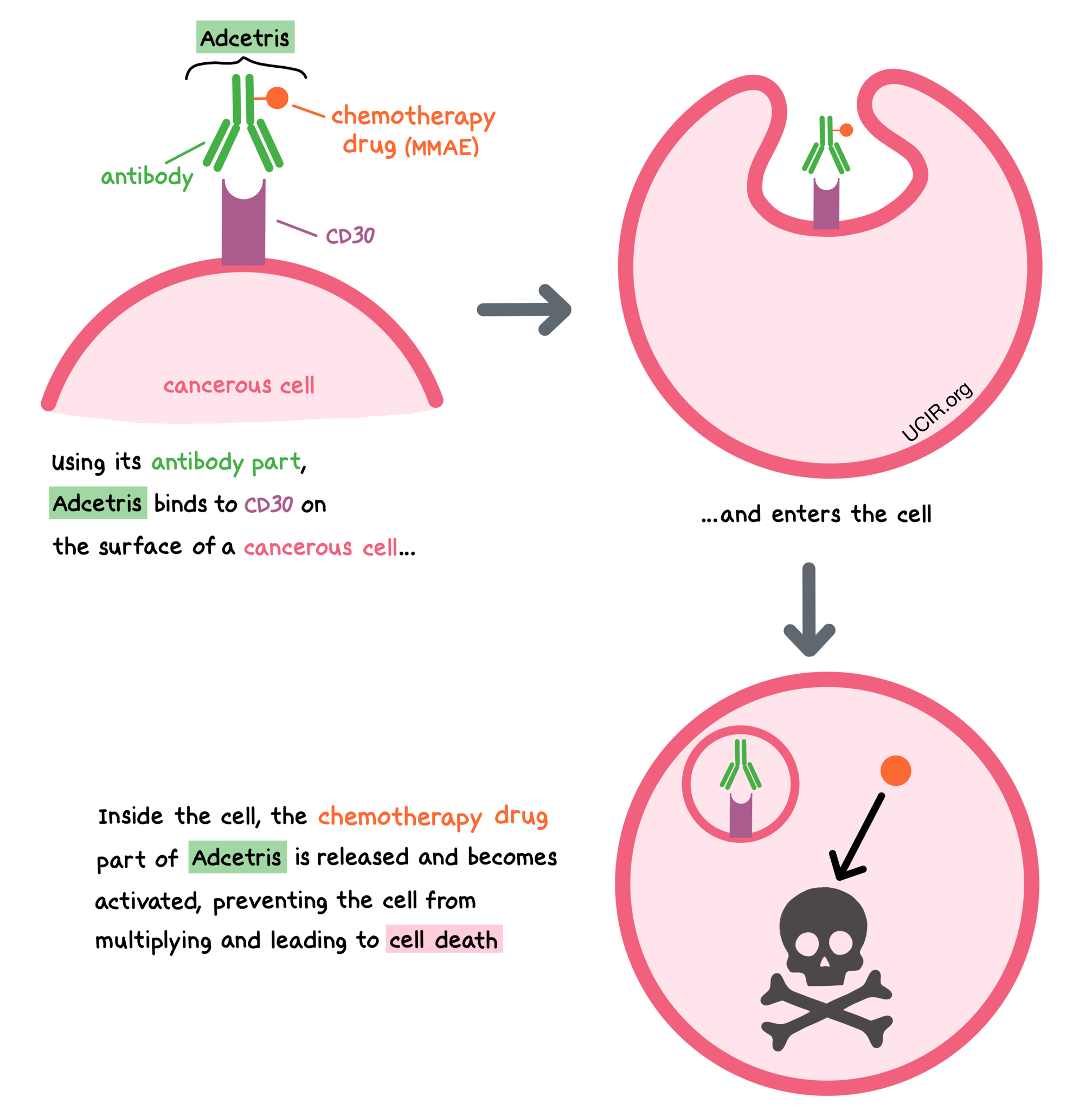
Adcetris is a medicine that consists of two parts that are connected: an antibody that attaches to a molecule called CD30 on the surface of cancer cells, and a chemotherapy drug called monomethyl auristatin E (MMAE).
CD30 is present on the surface of cancer cells in Hodgkin lymphoma and certain T cell lymphomas, and is also present in smaller amounts on the surface of some healthy B cells and T cells. CD30 plays a role in cell survival and multiplication.
By targeting CD30, Adcetris is designed to minimize harm to normal, healthy cells, and to bring the chemotherapy directly to the cancer cells. When Adcetris binds to CD30, it can enter the cell it is bound to. Inside the cell, the chemotherapy part of Adcetris is released and becomes activated. MMAE prevents the cancer cells from multiplying and leads to their death.
How is this drug given to the patient?
Prior to receiving Adcetris, patients may receive G-CSF (Figrastim), a treatment that helps prevent low white blood cell counts. Patients may also receive medications to help prevent nausea and vomiting.
Adcetris is administered via a tube into a vein (intravenous infusion, or I.V.) over 30 minutes, every two or three weeks (depending on the cancer type) and does not require a hospital stay. The maximum number of doses varies by cancer type.
What are the observed clinical results?
For:
Classical Hodgkin lymphoma (previously untreated)
Classical Hodgkin lymphoma (treated with stem cell transplant)
T cell lymphoma (previously untreated)
T cell lymphoma (previously treated)
Cutaneous T cell lymphoma (previously treated)
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Classical Hodgkin lymphoma (previously untreated)
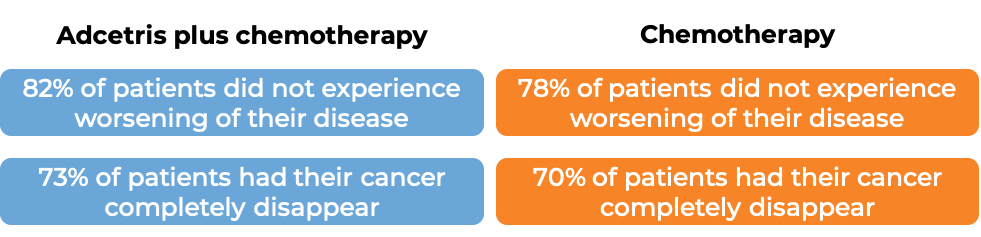
In a clinical trial, 1334 patients with previously untreated Stage III or IV classical Hodgkin lymphoma were treated with either:
- Adcetris plus chemotherapy (doxorubicin, vinblastine, and dacarbazine), OR
- chemotherapy alone (doxorubicin, vinblastine, dacarbazine, and bleomycin).
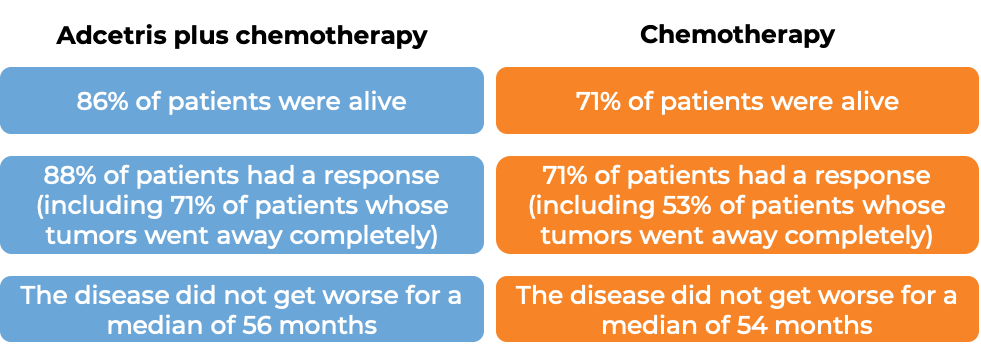
At a median follow-up of 25 months:
In another clinical trial, 600 young adult and pediatric patients 2 years and older (2 to <22 years of age) with previously untreated classical Hodgkin's lymphoma who were at high risk of their disease becoming worse were treated with either:
- Adcetris + AVEPC (Doxorubicin [A], Vincristine [V], Etoposide [E], Prednisone [P], Cyclophosphamide [C]), OR
- ABVE-PC (Doxorubicin [A], Bleomycin [B], Vincristine [V], Etoposide [E], Prednisone [P], Cyclophosphamide [C])
At a median follow-up of 42 months, 92% of patients who were treated with Adcetris + AVEPC, compared to 83% of patients treated with ABVE-PC, experienced some clinical benefit. The remaining patients (8% and 17%, respectively) experienced worsening or return of their disease, development of another cancer, or death of any cause.
Classical Hodgkin lymphoma (treated with stem cell transplant)
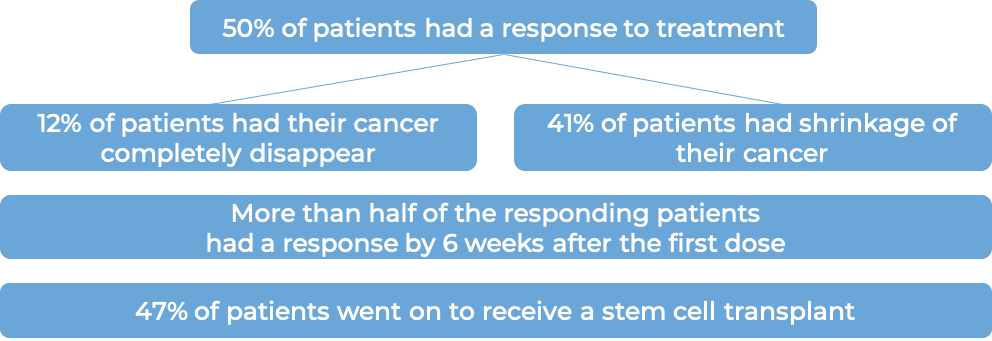
In a clinical trial, 329 patients with classical Hodgkin lymphoma who had received a stem cell transplant and were at a high risk of their disease coming back or becoming worse, were treated with either placebo or Adcetris to prevent the cancer from coming back. At a median follow-up of 22 months, the disease did not get worse for a median of 43 months for patients receiving Adcetris, compared with 24 months for patients receiving placebo.
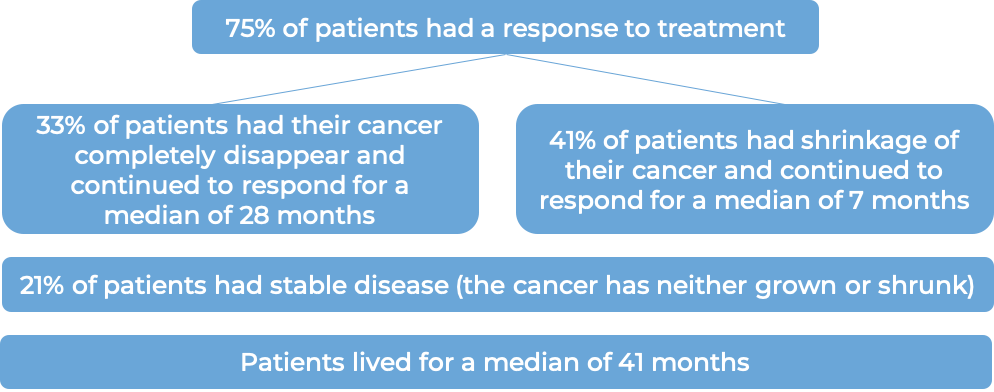
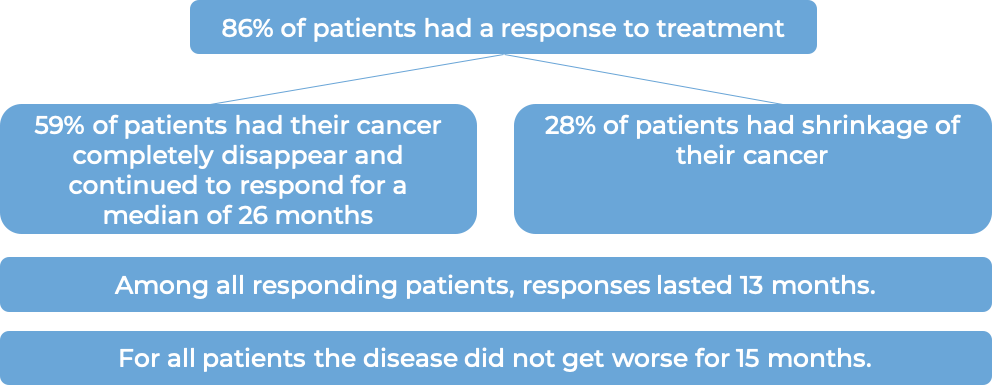
In another clinical trial, 102 patients with classical Hodgkin lymphoma who had received a stem cell transplant and the cancer came back, were treated with Adcetris. Patients saw a response to treatment at a median of 6 weeks:
T cell lymphoma (previously untreated)
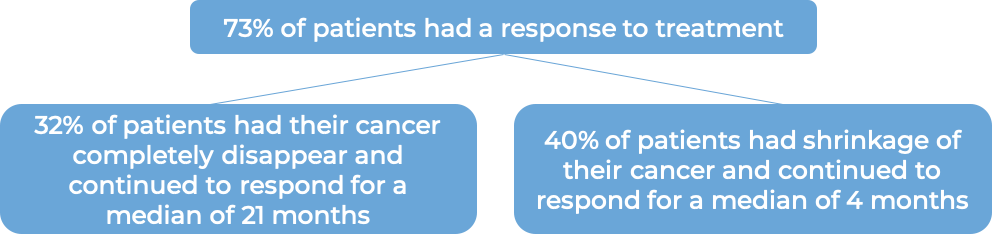
In a clinical trial, 452 patients with previously untreated systemic anaplastic large cell lymphoma (sALCL) or other peripheral T cell lymphomas that test positive for the CD30 molecule were treated with either:
- Adcetris plus chemotherapy (cyclophosphamide and doxorubicin) and prednisone, OR
- chemotherapy (cyclophosphamide, doxorubicin, and vincristine) and prednisone.
At a median follow-up of 42 months:
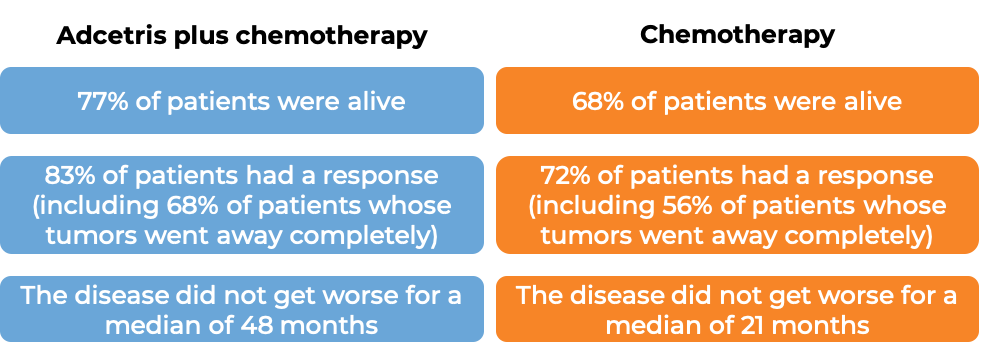
T cell lymphoma (previously treated)
In a clinical trial, 58 patients with systemic anaplastic large cell lymphoma (sALCL) who had received at least one combination chemotherapy, and the cancer came back, were treated with Adcetris. At a median follow-up of 16 months:
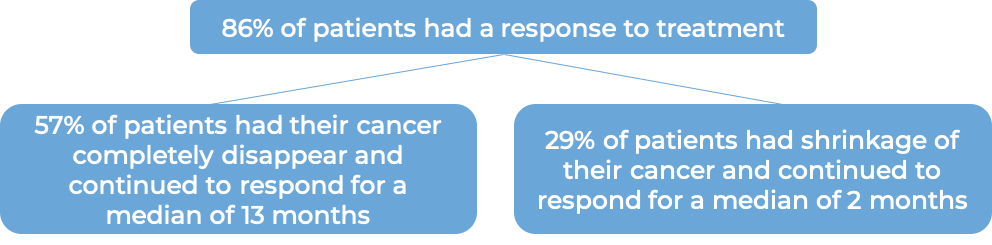
Cutaneous T cell lymphoma (previously treated)
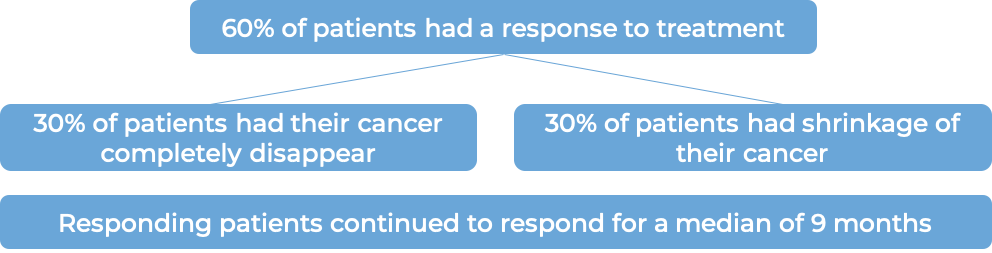
In a clinical trial, 131 patients with primary cutaneous anaplastic large cell lymphoma (pcALCL) or mycosis fungoides (MF) that tested positive for the CD30 molecule, who had previously received systemic therapy (treatment that affects the entire body) for their disease, were treated with either Adcetris or physician’s choice of chemotherapy (methotrexate or bexarotene). At a median follow-up of 23 months:
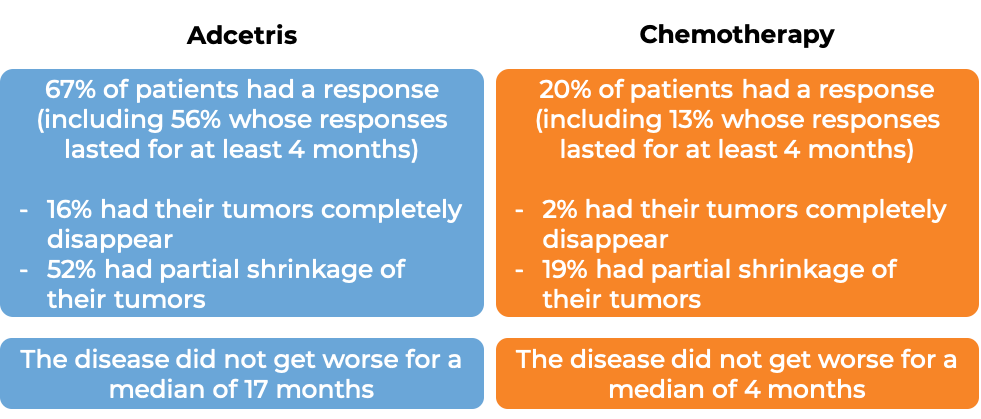
What are the side effects?
The most common side effects of Adcetris include nerve damage, tiredness, nausea, vomiting, diarrhea, constipation, weight loss, hair loss, fever, abdominal pain, muscle pain, difficulty breathing, infection in the nose or sinuses, sores or swelling in the mouth and/or in the digestive tract, low white blood cell counts, and low red blood cell counts.
Adcetris can cause side effects that can become serious or life-threatening and may lead to death. Some of the serious side effects related to Adcetris include nerve damage; problems with the lungs, pancreas, skin, and gastrointestinal tract (including developing a hole in the stomach or intestine); high blood sugar; low white blood cell counts; low red blood cell counts; low number of platelets; serious infections; liver damage; and tumor lysis syndrome (when large amounts of cancer cells are killed at the same time, which could lead to kidney failure). Allergic reactions and other reactions related to the infusion may occur.
Progressive Multifocal Leukoencephalopathy (PML)
PML is a rare, serious brain infection that is caused by a virus and can lead to death. Individuals with weakened immune systems have an increased risk of infection. Symptoms of PML include mood changes, confusion, dizziness or loss of balance, difficulty walking or talking, weakness on one side of the body, and vision problems.
Patients should immediately report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Seattle Genetics (US)
Takeda (Europe)
Approval
FDA and EMA
Links to drug websites
Other references
- Results of a Pivotal Phase II Study of Brentuximab Vedotin for Patients with Relapsed or Refractory Hodgkin’s Lymphoma. Yunes A. et al. Journal of Clinical Oncology (2012
- Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Prince M.H. et al. The Lancet (2017)
- Brentuximab Vedotin with Chemotherapy in Pediatric High-Risk Hodgkin’s Lymphoma. Catellinio S.M. et al. The New England Journal of Medicine (2022)
Last updated on March 14, 2024