How is this drug name pronounced?
Bevacizumab: beh-vuh-SIH-zoo-mab
Avastin: uh-VAS-tin
Although Avastin was the first approved bevacizumab drug, there are now two approved drugs that are “biosimilar” to Avastin: Mvasi (bevacizumab-awwb) and Zirabev (bevacizumab-bvzr). Mvasi and Zirabev have been shown to be similar to Avastin in the way in which they work, how well they work, and how safe they are.
What cancer(s) does this drug treat?
Avastin has been approved for a number of cancer types and stages, in some cases as a single therapy, and in some cases in combination with other therapies.
Avastin is approved for:
Colorectal cancer
Non-small cell lung cancer
Glioblastoma
Kidney cancer
Cervical cancer
Ovarian, fallopian tube, or primary peritoneal cancer
Liver cancer
Advanced colorectal cancer
Avastin is approved for:
- Patients with colon or rectal cancer that has spread to other parts of the body. In such cases, Avastin is used in combination with intravenous chemotherapy containing fluorouracil as a first treatment for patients with previously untreated advanced disease, or as a second treatment for patients with previously treated advanced disease.
- Patients with colon or rectal cancer that has spread and who have received treatment containing bevacizumab (Avastin, Mvasi, or Zirabev) and chemotherapy, and the treatment did not work or stopped working. In such cases, Avastin is used in combination with chemotherapy containing fluoropyrimidine and either irinotecan (Onivyde or Camptosar) or oxaliplatin (Eloxatin), depending on the chemotherapy initially used.
Advanced non-small cell lung cancer
Avastin is approved for:
- Patients with nonsquamous non-small cell lung cancer that has grown, come back, or spread and cannot be removed by surgery, and who have not received chemotherapy for their advanced disease. In such cases, Avastin is used in combination with paclitaxel (Taxol or Onxal) and carboplatin (Paraplatin) as a first treatment.
Glioblastoma
Avastin is approved for:
- Patients with glioblastoma that has come back or gotten worse after previous treatment.
Advanced kidney cancer
Avastin is approved for:
- Patients with advanced renal cell carcinoma (kidney cancer) that has spread. In such cases, Avastin is used in combination with interferon alfa.
Advanced cervical cancer
Avastin is approved for:
- Patients with cervical cancer that has not responded to previous treatment, has come back, or has spread. In such cases, Avastin is used in combination with paclitaxel (Taxol or Onxal) and either cisplatin (Platinol) or topotecan (Hycamtin).
Advanced ovarian, fallopian tube, or primary peritoneal cancer
Avastin is approved for:
-
Patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer:
- For Stage III or IV disease after initial surgery, Avastin is used in combination with paclitaxel (Taxol or Onxal) and carboplatin (Paraplatin), followed by Avastin alone.
- For patients who have received no more than two prior chemotherapy treatments, and whose disease is resistant to chemotherapy containing platinum (disease initially responded to treatment, but came back within 6 months), Avastin is used in combination with paclitaxel (Taxol or Onxal), pegylated liposomal doxorubicin (Doxil or Caelyx), or topotecan (Hycamtin).
- For patients with disease that has come back, and whose disease is sensitive to chemotherapy containing platinum (disease initially responded to treatment, but came back more than 6 months later), Avastin is used in combination with carboplatin (Paraplatin) and either paclitaxel (Taxol or Onxal) or gemcitabine (Gemzar), followed by Avastin alone.
Advanced liver cancer
Avastin is approved for:
- Patients with hepatocellular carcinoma (liver cancer) that has spread or cannot be removed by surgery, and who have not received prior systemic therapy (therapy by mouth or injection into the vein [I.V.]) for their disease. In such cases, Avastin is used in combination with atezolizumab (Tecentriq).
Limitations of use:
Age: The safety and efficacy of Avastin in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Avastin can cause ovaries to stop working and impair the ability to have children. Avastin can cause harm to a fetus, and is not recommended for use during pregnancy. Pregnancy should be prevented for at least six months after the last dose of Avastin. The risks associated with Avastin during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for at least six months after the last dose of Avastin.
What type of immunotherapy is this?
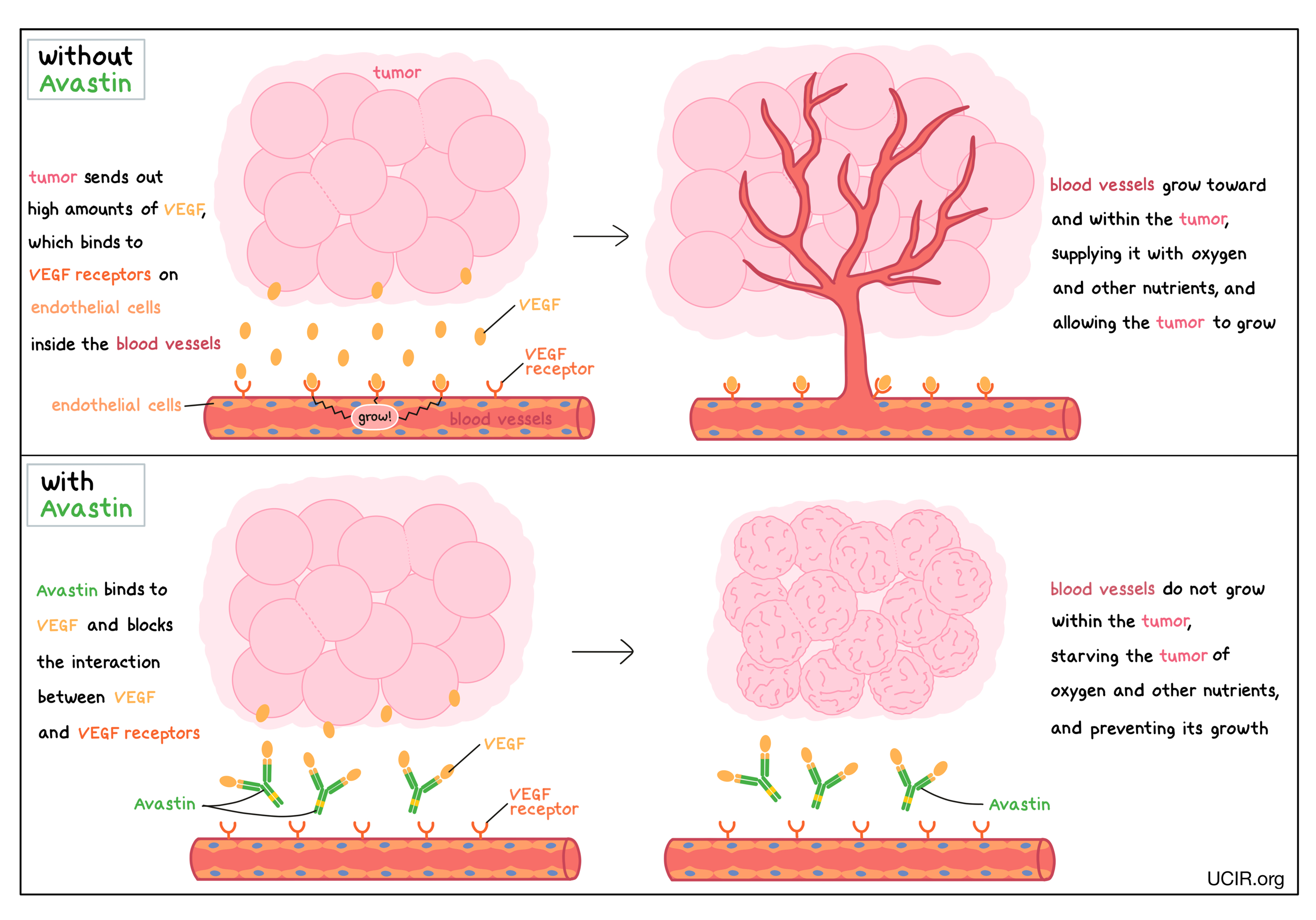
How does this drug work?
- Target: vascular endothelial growth factor (VEGF)
Avastin is an antibody that attaches to a protein molecule called vascular endothelial growth factor (VEGF). VEGF stimulates the formation of new blood vessels and promotes growth of preexisting blood vessels – a process called angiogenesis. In a healthy body, VEGF is produced by many different types of cells and helps to create new blood vessels during embryonic development, normal growth of tissues, and wound healing. Tumors make higher than normal amounts of VEGF because the formation of new blood vessels within the tumor is critical for the cancer to grow (by providing the tumor with access to oxygen and other nutrients) and to spread to other parts of the body (by providing a route for cancer cells to enter blood circulation and move to other locations). VEGF molecules produced by tumors bind to molecules called VEGF receptors that are present on cells found in the inside lining of blood vessels (endothelial cells). The binding of VEGF to VEGF receptors leads the endothelial cells to multiply, migrate to the source of the VEGF molecules (the tumor site), and form new blood vessels within the tumor.
Avastin binds to the VEGF molecules within blood vessels in a way that blocks the interaction between VEGF and the VEGF receptors on endothelial cells. This helps prevent the formation of new blood vessels within the tumor, thus starving the tumor of oxygen and other nutrients, and preventing its growth.
How is this drug given to the patient?
Avastin is administered via a tube into a vein (intravenous infusion, or I.V.) every two or three weeks (depending on the cancer type) and does not require a hospital stay. The first infusion is administered over 90 minutes. If the first infusion is tolerated, the second infusion is administered over 60 minutes. If the second infusion is tolerated, all following infusions are administered over 30 minutes.
Avastin should not be administered for at least 28 days after surgery, and only after the surgical wound is fully healed.
What are the observed clinical results?
For:
Advanced colorectal cancer (previously untreated or treated)
Advanced non-small cell lung cancer
Glioblastoma
Advanced kidney cancer
Advanced cervical cancer
Advanced ovarian, fallopian tube, or primary peritoneal cancer (Stage III or IV disease after initial surgery)
Advanced ovarian, fallopian tube, or primary peritoneal cancer (platinum-resistant)
Advanced ovarian, fallopian tube, or primary peritoneal cancer (platinum-sensitive)
Advanced liver cancer
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced colorectal cancer (previously untreated or treated)
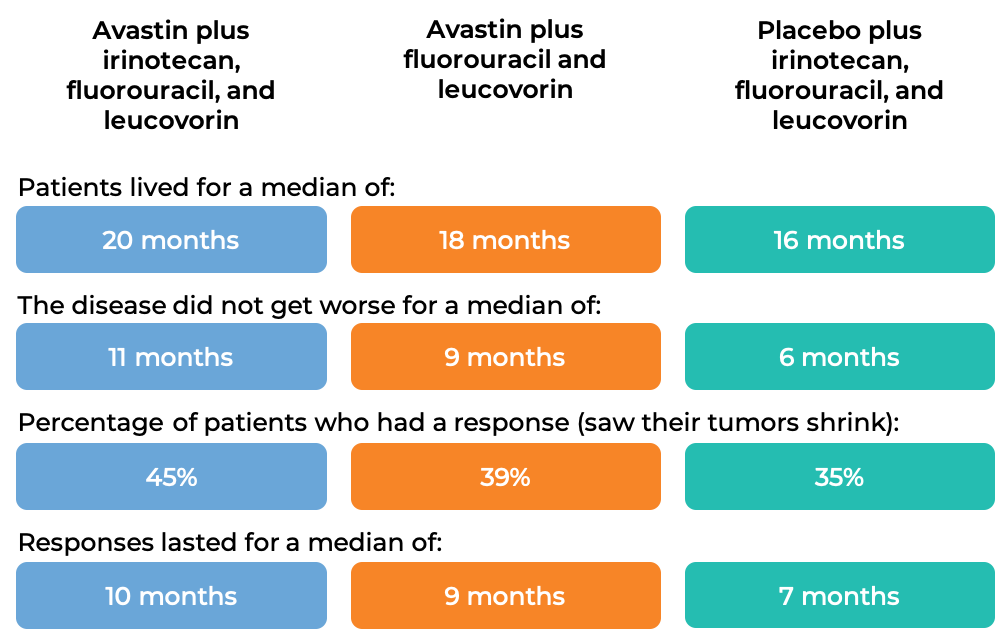
In a clinical trial, 923 patients with previously untreated advanced colorectal cancer that had spread were treated with:
- Avastin plus irinotecan (Onivyde or Camptosar), fluorouracil, and leucovorin, OR
- Avastin plus fluorouracil and leucovorin, OR
- Placebo plus irinotecan, fluorouracil, and leucovorin.
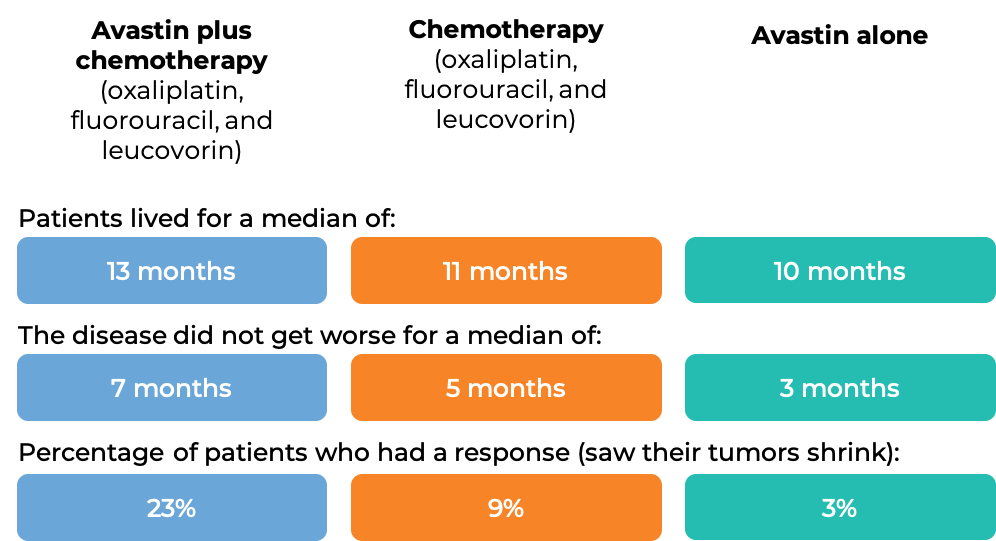
In another clinical trial, 829 patients with advanced colorectal cancer that had spread, and who had been previously treated with irinotecan (Onivyde or Camptosar) and fluorouracil, were treated with:
- Avastin plus oxaliplatin (Eloxatin), fluorouracil, and leucovorin, OR
- oxaliplatin, fluorouracil, and leucovorin, OR
- Avastin alone.
At a median follow-up of 28 months:
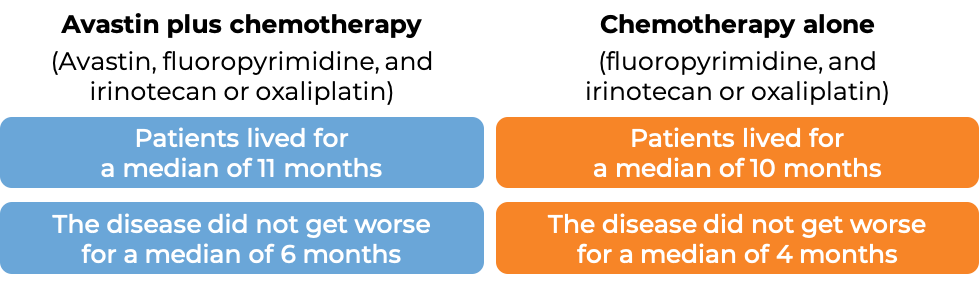
In another clinical trial, 820 patients with advanced colorectal cancer that had spread, who had previously received Avastin-containing treatment, and it either did not work or stopped working, were treated with either Avastin plus chemotherapy (fluoropyrimidine with either irinotecan (Onivyde or Camptosar) or oxaliplatin (Eloxatin)) or chemotherapy alone.
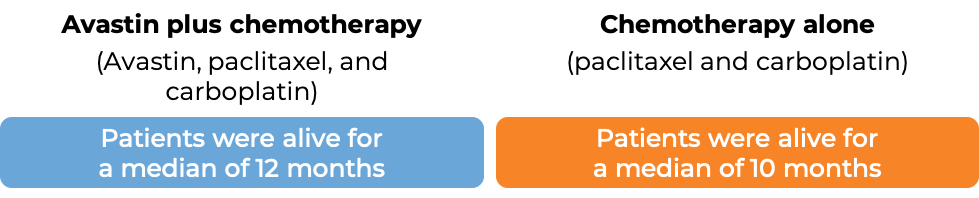
Advanced non-small cell lung cancer
In a clinical trial, 878 patients with nonsquamous non-small cell lung cancer that had grown, spread, or come back, and who had not received chemotherapy for their advanced disease, were treated with either Avastin plus chemotherapy (paclitaxel (Taxol or Onxal) and carboplatin (Paraplatin)) or chemotherapy (paclitaxel and carboplatin) alone.
Glioblastoma
In a clinical trial, 432 patients with recurrent glioblastoma (glioblastoma that had come back or gotten worse after previous treatment) were treated with either Avastin in combination with the chemotherapy drug lomustine (Gelostine) or with lomustine alone. The disease did not get worse for a median of 4 months for patients treated with Avastin plus lomustine, compared with 2 months for patients treated with lomustine alone. There was no difference in overall survival. Among patients requiring corticosteroids at the beginning of the study, 23% of patients receiving Avastin plus lomustine were able to stop taking corticosteroids, compared with 12% of patients receiving lomustine alone.
In two other clinical trials, patients with previously treated glioblastoma were treated with Avastin. In one trial, 26% of 85 patients responded to treatment, and patients continued to respond for a median of 4 months. In the other trial, 20% of 56 patients responded to treatment (saw their cancer shrink), and patients continued to respond for a median of 4 months.
Advanced kidney cancer
In a clinical trial, 649 patients with previously untreated advanced renal cell carcinoma (kidney cancer) that had spread were treated with either Avastin and interferon alfa or placebo and interferon alfa. For patients treated with Avastin and interferon alfa, the disease did not get worse for a median of 10 months, compared with 5 months for patients treated with placebo and interferon alfa. Of the 595 patients with measurable disease, 31% of patients treated with Avastin and interferon alfa responded to treatment (saw their cancer shrink), compared with 12% of patients responding to placebo and interferon alfa.
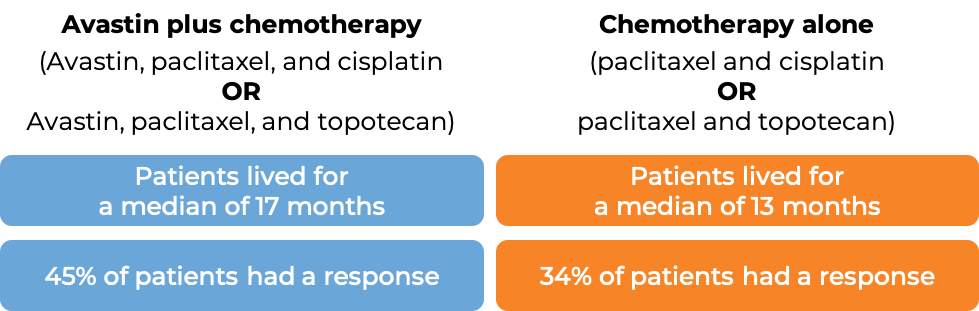
Advanced cervical cancer
In a clinical trial, 452 patients with cervical cancer that had not responded to previous treatment, had come back, or had spread, were treated with:
- Avastin with chemotherapies paclitaxel (Taxol or Onxal) and cisplatin (Platinol), OR
- Avastin with chemotherapies paclitaxel and topotecan (Hycamtin), OR
- chemotherapies paclitaxel and cisplatin, OR
- chemotherapies paclitaxel and topotecan.
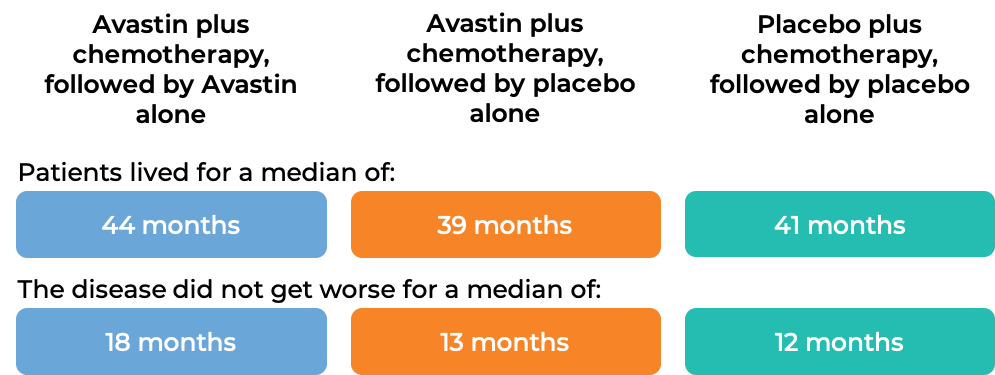
Advanced ovarian, fallopian tube, or primary peritoneal cancer (Stage III or IV disease after initial surgery)
In a clinical trial, 1873 patients with Stage III or IV epithelial ovarian, fallopian tube, or primary peritoneal cancer, who had undergone initial surgery, were treated with:
- Avastin plus chemotherapy (paclitaxel (Taxol or Onxal) and carboplatin (Paraplatin)), followed by Avastin alone, OR
- Avastin plus chemotherapy (paclitaxel and carboplatin), followed by placebo alone, OR
- placebo plus chemotherapy (paclitaxel and carboplatin), followed by placebo alone.
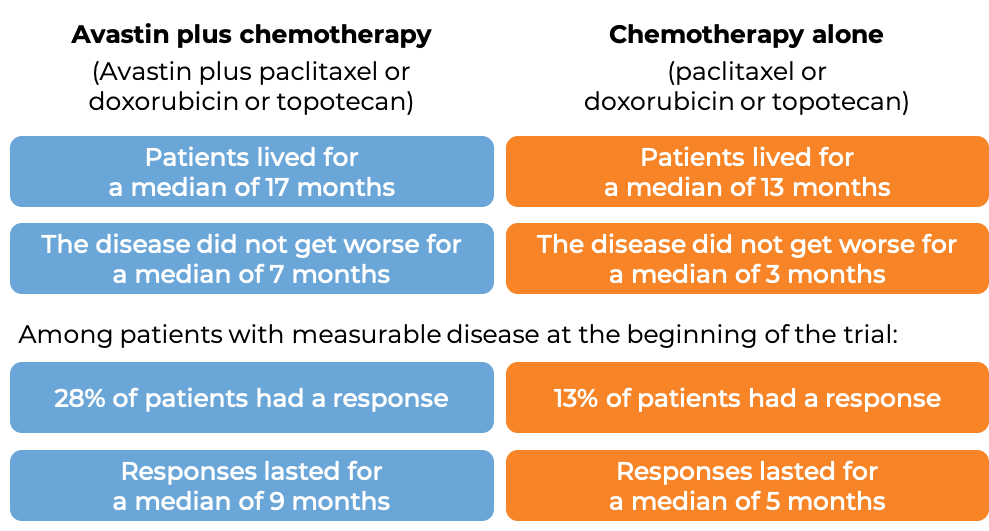
Advanced ovarian, fallopian tube, or primary peritoneal cancer (platinum-resistant)
In a clinical trial, 361 patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer, whose disease was resistant to chemotherapy containing platinum (disease responded to treatment, but came back within 6 months), were treated with either Avastin with chemotherapy (investigator’s choice of paclitaxel (Taxol or Onxal), pegylated liposomal doxorubicin (Doxil or Caelyx), or topotecan (Hycamtin))or chemotherapy alone.
Advanced ovarian, fallopian tube, or primary peritoneal cancer (platinum-sensitive)
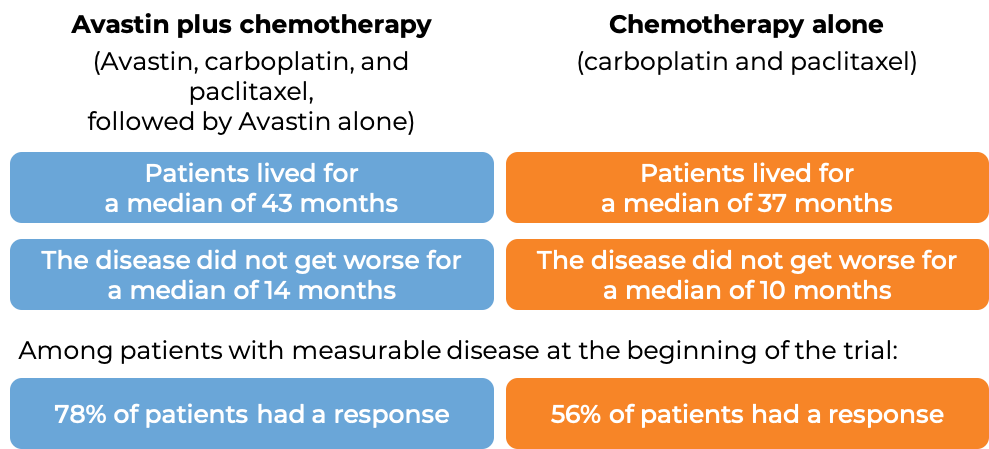
In a clinical trial, 484 patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer, whose disease was sensitive to chemotherapy containing platinum, but then came back (disease responded to treatment, but came back more than 6 months later), who had not received chemotherapy once the disease returned, and who had not previously received Avastin, were treated with:
- Avastin plus chemotherapy (carboplatin (Paraplatin) and gemcitabine (Gemzar)), followed by Avastin alone, OR
- Placebo plus chemotherapy (carboplatin and gemcitabine), followed by placebo alone.
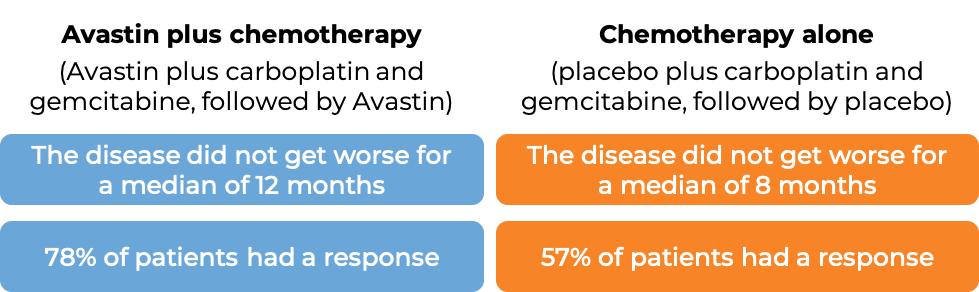
In another clinical trial, 673 patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer whose disease was sensitive to chemotherapy containing platinum, but then came back more than 6 months later, and who had not received more than one course of chemotherapy, were treated with:
- Avastin plus chemotherapy (carboplatin (Paraplatin) and paclitaxel (Taxol or Onxal)), followed by Avastin alone, OR
- Chemotherapy (carboplatin and paclitaxel).
Advanced liver cancer
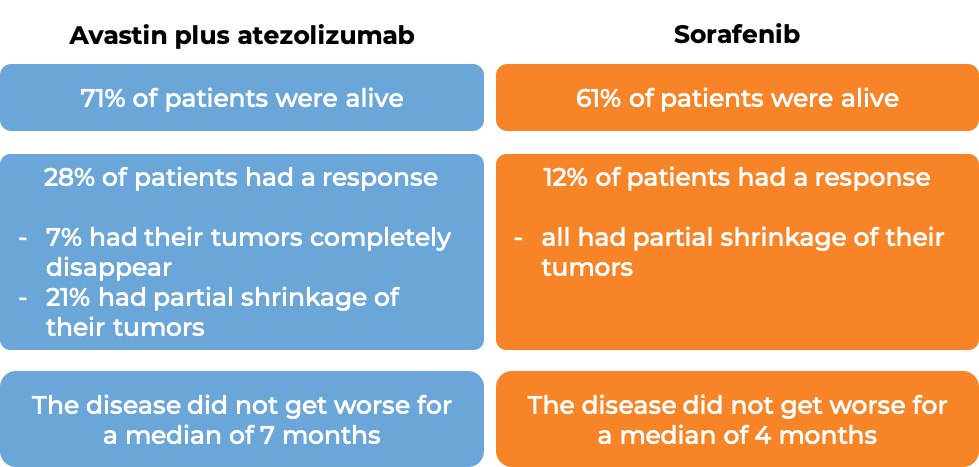
In a clinical trial, 501 patients with hepatocellular carcinoma (liver cancer) that had spread or could not be removed by surgery, and who had not received prior systemic therapy (therapy by mouth or injection into the vein [I.V.]) for their disease, were treated with either Avastin plus atezolizumab (Tecentriq) OR sorafenib (Nexavar). At a median follow-up of 9 months:
What are the side effects?
The most common side effects of Avastin include high blood pressure, nosebleeds, rectal bleeding, diarrhea, abdominal (belly) pain, back pain, headache, fatigue, weakness, taste change, dry skin, inflammation of the skin, inflammation of the nose, watery eyes, and too much protein in the urine.
Avastin can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Avastin include development of a hole in the stomach or intestine, formation of an abnormal connection between two body parts (a fistula), blood clots, serious bleeding, having wounds that do not heal, infections, fever, and severe high blood pressure. Avastin should not be administered 28 days prior and following any major surgery or until the wounds from surgery have fully healed. Additionally, problems can arise with the kidneys, the heart, the nervous system, and vision. Reactions related to the infusion may also occur. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Genentech
Approval
FDA and EMA
Links to drug websites
Other references
- The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Tabernero J. Molecular Cancer Research (2007)
- Bevacizumab in Combination With Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results From the Eastern Cooperative Oncology Group Study E3200. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Journal of Clinical Oncology (2016)
- Paclitaxel–Carboplatin Alone or with Bevacizumab for Non–Small-Cell Lung Cancer. Sandler A, Gray R, Perry MC, et al. The New England Journal of Medicine (2006)
- Avastin (bevacizumab), for U.S. healthcare professionals. Accessed February 3, 2020.
Last updated June 2, 2021


