How is this drug name pronounced?
Atezolizumab: A-teh-zoh-LIZ-yoo-mab
Tecentriq: teh-SEN-trik
What cancer(s) does this drug treat?
Tecentriq has been approved for a number of cancer types and stages, in some cases as a single therapy and in some cases in combination with other therapies.
Tecentriq is approved for:
Advanced non-small cell lung cancer
Small cell lung cancer
Liver cancer
Melanoma
Sarcoma
Non-small cell lung cancer
Tecentriq is approved for:
- Patients with non-small cell lung cancer that has been removed by surgery and treated with platinum-based chemotherapy, and tests positive for high levels of the PD-L1 molecule.
- Patients with nonsquamous non-small cell lung cancer that has spread and does not have an abnormal EGFR or ALK gene. In such cases, Tecentriq may be used in combination with bevacizumab (Avastin, Mvasi, Zirabev), paclitaxel (Taxol or Onxal), and carboplatin (Paraplatin) as a first treatment. Alternatively, Tecentriq may be used in combination with paclitaxel protein-bound (Abraxane) and carboplatin (Paraplatin) as a first treatment.
- Patients with non-small cell lung cancer that has spread, whose tumors test positive for high levels of the PD-L1 molecule, and whose tumors do not have an abnormal EGFR or ALK gene. In such cases, Tecentriq is used as a first treatment.
- Patients with non-small cell lung cancer that has spread. The patient must have already tried chemotherapy containing platinum and it either did not work or stopped working. If the patient’s tumor has an abnormal EGFR or ALK gene, the patient has to have also tried therapy for tumors with such abnormal genes prior to receiving Tecentriq.
Advanced small cell lung cancer
Tecentriq is approved for:
- Patients with extensive-stage small cell lung cancer that has grown or spread. In such cases, Tecentriq may be used in combination with chemotherapies carboplatin (Paraplatin) and etoposide (Etopophos) as a first treatment.
Advanced liver cancer
Tecentriq is approved for:
- Patients with liver cancer (hepatocellular carcinoma) that could not be removed by surgery or has spread to other parts of the body, and who have not yet received systemic therapy (therapy by mouth or injection into the vein [i.v.]) for their disease. In such cases, Tecentriq is used in combination with bevacizumab (Avastin, Mvasi, Zirabev).
Advanced melanoma
Tecentriq is approved for:
- Patients with melanoma that could not be removed by surgery or had spread to other parts of the body, and who tested positive for the BRAF V600 mutation. In such cases, Tecentriq is given in combination with cobimetinib (Cotellic) and vemurafenib (Zelboraf).
Alveolar soft part sarcoma
Tecentriq is approved for:
- Adult and pediatric patients (2 years and older) with alveolar soft part sarcoma that cannot be removed by surgery or has spread to other parts of the body.
Limitations of use:
Age: The safety and efficacy of Tecentriq have been established in pediatric patients with alveolar soft part sarcoma 2 years of age or older, but not in patients less than 2 years old. The safety and efficacy of Tecentriq in patients under 18 years of age have not been established for any other indications.
Fertility/Pregnancy/Breastfeeding: Tecentriq may impair fertility in women while receiving treatment. Tecentriq can cause harm to a fetus, and is not recommended for use during pregnancy. Pregnancy should be prevented for at least five months after the last dose of Tecentriq. The risks associated with Tecentriq during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for at least five months after the last dose of Tecentriq.
Complications of stem cell transplant: Serious and life-threatening complications that can lead to death can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Tecentriq.
What type of immunotherapy is this?
How does this drug work?
Tecentriq is an antibody that attaches to a molecule called PD-L1, which is sometimes present on the surface of cancer cells or other cells within the tumor mass. PD-L1 interacts with a molecule called PD-1, which is present on the surface of T cells– the primary immune cells involved in killing cancer cells. In healthy tissues, the interaction between PD-L1 and PD-1 puts on a brake that keeps T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from doing their job – killing the cancer cells. When PD-L1 interacts with PD-1 on T cells, the T cells become inactive and do not attack the cancer cells. Tecentriq binds to the PD-L1 molecules on cancer cells and other cells within the tumor mass in such a way that it blocks the interaction between PD-1 and PD-L1, and allows the T cells to be active and attack the cancer cells.
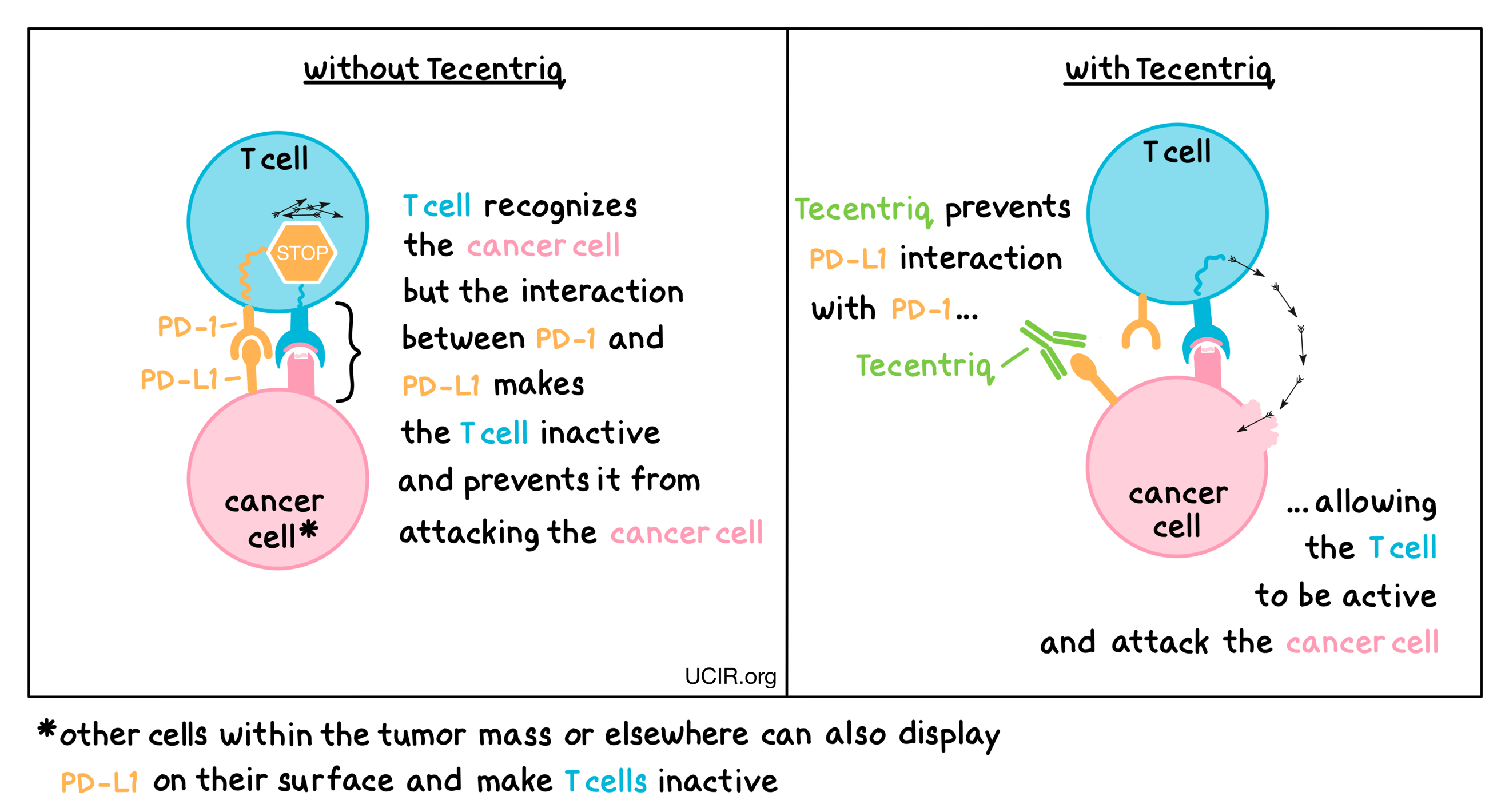
How is this drug given to the patient?
Tecentriq is administered via a tube into a vein (intravenous infusion, or i.v.) over 60 minutes every two, three, or four weeks (depending on the dose) and does not require a hospital stay. If the first infusion is tolerated, all following infusions may be administered over 30 minutes.
What are the observed clinical results?
For:
Non-small cell lung cancer (removed by surgery)
Advanced non-small cell lung cancer (previously untreated with chemotherapy)
Advanced non-small cell lung cancer (previously treated with chemotherapy)
Advanced small cell lung cancer
Advanced liver cancer
Advanced melanoma
Alveolar soft part sarcoma
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Non-small cell lung cancer (removed by surgery)
In a clinical trial, 1005 patients with non-small cell lung cancer were either treated with Tecentriq or best supportive care following complete surgical removal of all known disease and cisplatin-based chemotherapy. Among 476 patients whose tumor tested positive for the PD-L1 molecule, at a median follow-up of 32 months:

Advanced non-small cell lung cancer (previously untreated with chemotherapy)
In a clinical trial of patients with nonsquamous non-small cell lung cancer that had spread and who had not previously been treated with chemotherapy for their advanced disease, patients were treated with one of the following three treatment options:
- Treatment 1: Tecentriq in combination with paclitaxel (Taxol or Onxal) and carboplatin (Paraplatin)
- Treatment 2: Tecentriq in combination with bevacizumab (Avastin), paclitaxel (Taxol or Onxal), and carboplatin (Paraplatin)
- Treatment 3: bevacizumab (Avastin), paclitaxel (Taxol or Onxal), and carboplatin (Paraplatin)
In the 1045 of 1202 enrolled patients whose cancer did not have an abnormal EGFR or ALK gene, at a median follow-up of 20 months, the following results were observed for patients receiving either Treatment 2 or 3:
(Results for patients receiving Treatment 1 did not significantly differ from the results observed with Treatment 3, and the combination used in the Treatment 1 arm did not receive approval.)
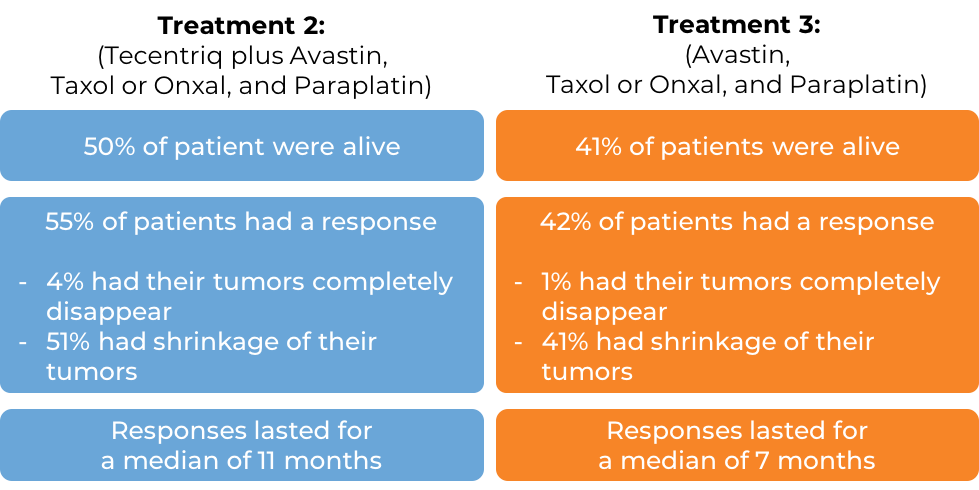
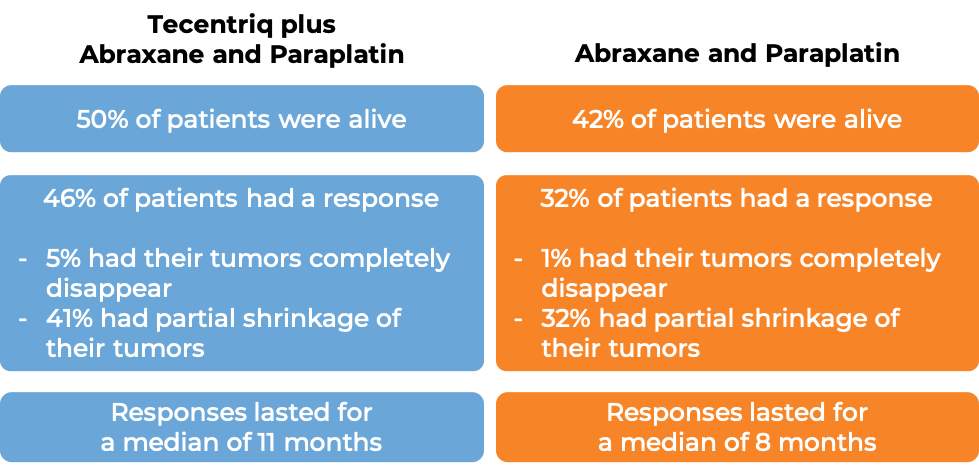
In another clinical trial of patients with nonsquamous non-small cell lung cancer that had spread and who had not previously been treated with chemotherapy for their advanced disease, patients were treated with either a combination of Tecentriq, paclitaxel protein-bound (Abraxane), and carboplatin (Paraplatin) or a combination of paclitaxel protein-bound (Abraxane) and carboplatin (Paraplatin). In the 681 of 724 enrolled patients whose cancer did not have an abnormal EGFR or ALK gene:
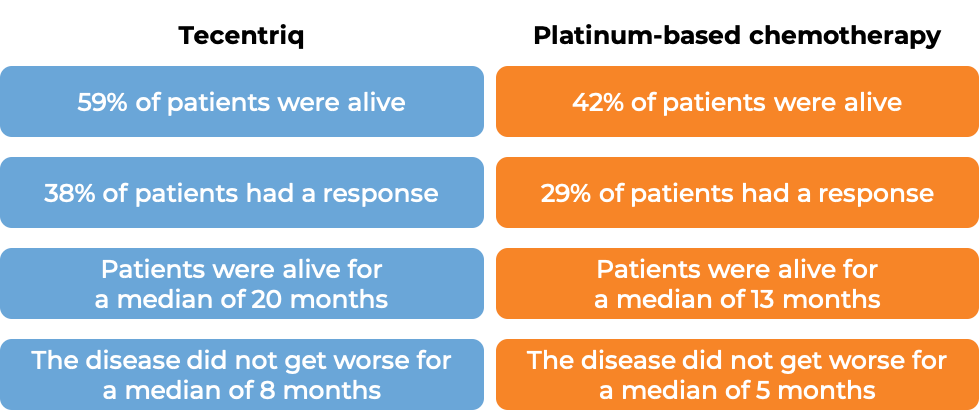
In another clinical trial, 205 patients with non-small cell lung cancer that had spread, whose tumors tested positive for high levels of the PD-L1 molecule, and whose tumors did not have an abnormal EGFR or ALK gene, were treated with either Tecentriq or platinum-based chemotherapy as first therapy. At a median follow-up of 16 months:
Advanced non-small cell lung cancer (previously treated with chemotherapy)
In a clinical trial of patients with non-small cell lung cancer that had grown or spread and who had already tried chemotherapy containing platinum, but it either did not work or stopped working, patients were treated with either Tecentriq or docetaxel (a chemotherapy). In the first 850 treated patients, at a median follow-up of 21 months following treatment:
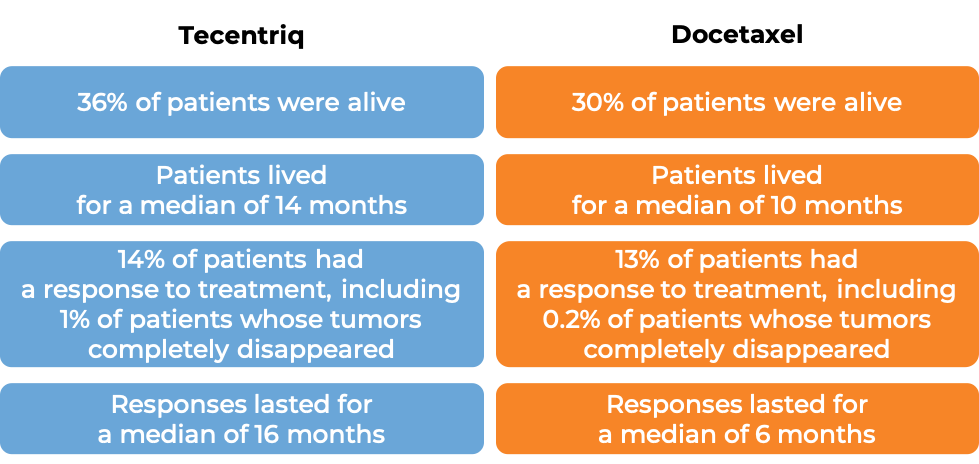
Advanced small cell lung cancer
In a clinical trial of 403 patients with extensive-stage small cell lung cancer that had grown or spread and who had not been treated with chemotherapy for their advanced disease, patients were treated with either Tecentriq in combination with the chemotherapies carboplatin (Paraplatin) and etoposide (Etopophos), or with a placebo in combination with carboplatin (Paraplatin) and etoposide (Etopophos). At a median follow-up of 14 months following treatment:
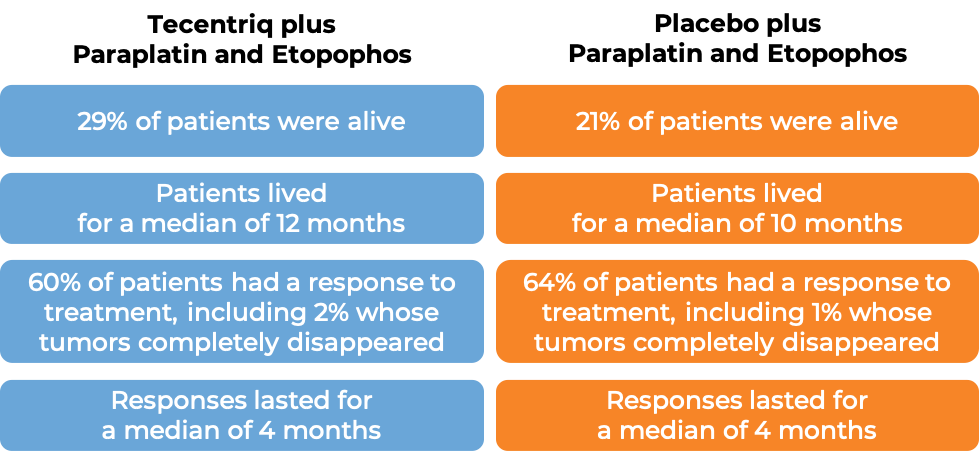
Advanced liver cancer
In a clinical trial, 501 patients with liver cancer (hepatocellular carcinoma) that could not be removed by surgery or had spread to other parts of the body, and who had not received systemic therapy (therapy by mouth or injection into the vein [I.V.]) for their disease, were treated with either Tecentriq plus bevacizumab (Avastin, Mvasi, Zirabev) OR sorafenib (Nexavar). At a median follow-up of 9 months:
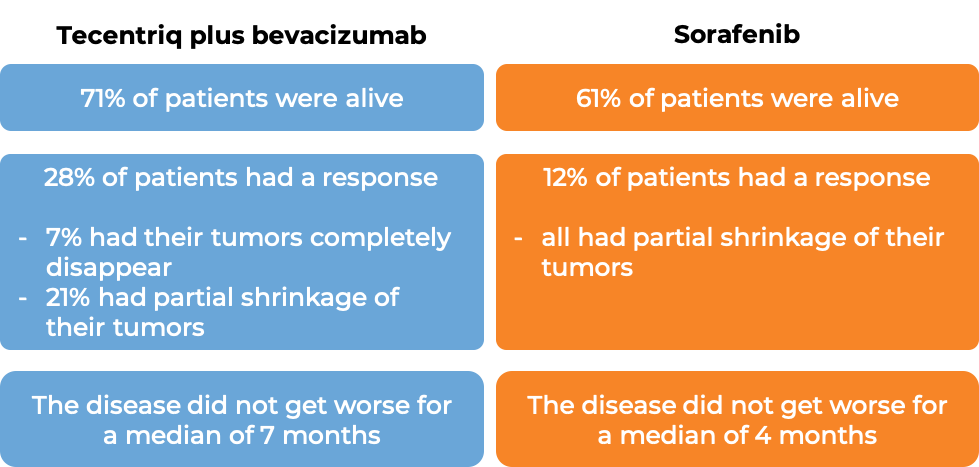
Advanced melanoma
In a clinical trial, 514 patients with melanoma that could not be removed by surgery or had spread to other parts of the body, and who tested positive for the BRAF V600 mutation, were treated with cobimetinib (Cotellic) and vemurafenib (Zelboraf) for 28 days and then received either Tecentriq plus cobimetinib and vemurafenib, or placebo plus cobimetinib and vemurafenib. At a median follow-up 19 months:
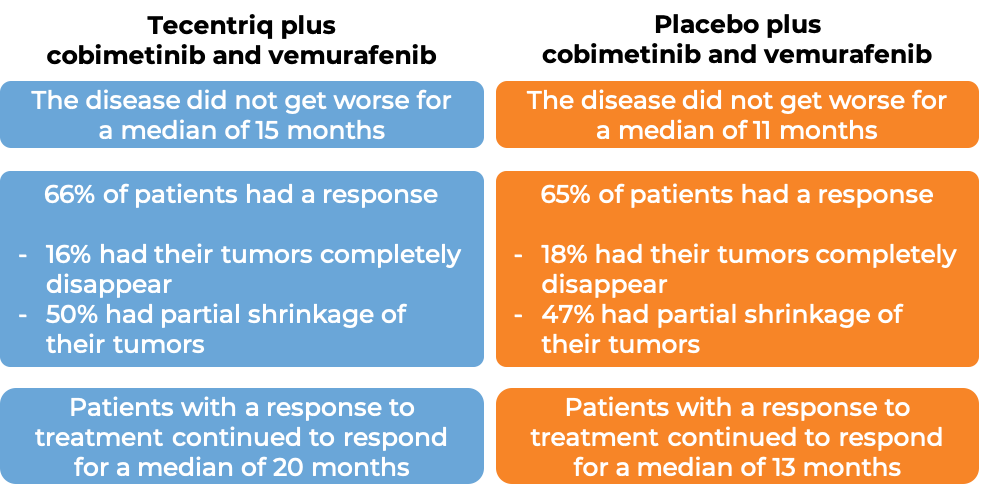
Alveolar soft part sarcoma
In a clinical trial, 49 adult and pediatric patients (2 years and older) with alveolar soft part sarcoma that could not be removed by surgery or had spread to other parts of the body, were treated with Tecentriq. 24% of patients saw shrinkage of their tumors. Of these patients, 67% continued to respond to therapy for more than 6 months and 47% experienced a response that lasted for 12 months or longer.
What are the side effects?
The most common side effects of Tecentriq include fatigue, weakness, nausea, decreased appetite, cough, and shortness of breath. When Tecentriq is used in combination with other drugs, other common side effects include diarrhea, constipation, vomiting, hair loss, headache, tingling or numbness in hands or feet, pain (in the muscles, bones, and joints), skin rash, heightened skin sensitivity to sunlight, low red blood cell count (anemia), and low white blood cell count.
Tecentriq can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Tecentriq can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Tecentriq include inflammation of the lungs, liver, or colon. Additionally, problems can arise with the heart, vision, and hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Tecentriq may cause Type 1 diabetes. Skin blisters and reactions related to the infusion may also occur. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Genentech
Approval
FDA and EMA
Links to drug websites
Other references
Last updated on December 20, 2022
How is this drug name pronounced?
Atezolizumab: A-teh-zoh-LIZ-yoo-mab
Tecentriq: teh-SEN-trik
What cancer(s) does this drug treat?
Tecentriq has been approved for a number of cancer types and stages, in some cases as a single therapy and in some cases in combination with other therapies.
Tecentriq is approved for:
Bladder and urinary tract (urothelial cell) cancer
Non-small cell lung cancer
Small cell lung cancer
Triple-negative breast cancer
Liver cancer
Advanced bladder and urinary tract (urothelial cell) cancer
Tecentriq is approved for:
Early-stage non-small cell lung cancer
Tecentriq is approved for:
Advanced non-small cell lung cancer
Tecentriq is approved for:
- Patients with nonsquamous non-small cell lung cancer that has spread. In such cases, Tecentriq may be used in combination with bevacizumab (Avastin, Mvasi, Zirabev), paclitaxel (Taxol or Onxal), and carboplatin (Paraplatin) as a first treatment. If the patient’s tumor has an abnormal EGFR or ALK gene, the patient has to have also tried therapy for tumors with such abnormal genes prior to receiving Tecentriq.
- Patients with nonsquamous non-small cell lung cancer that has spread and does not have an abnormal EGFR or ALK gene. In such cases, Tecentriq may be used in combination with protein-bound paclitaxel (nab-paclitaxel; Abraxane) and carboplatin (Paraplatin) as a first treatment.
- Patients with non-small cell lung cancer that has grown or spread. The patient must have already tried chemotherapy and it either did not work or stopped working. If the patient’s tumor has an abnormal EGFR or ALK gene, the patient has to have also tried therapy for tumors with such abnormal genes prior to receiving Tecentriq. In such cases, Tecentriq is used by itself.
- Patients with non-small cell lung cancer that has spread to other parts of the body, does not have an abnormal EGFR or ALK gene, and tests positive for high amounts of the PD-L1 molecule or immune cells within the tumor bed. In such cases, Tencentriq is given as a first treatment.
Advanced small cell lung cancer
Tecentriq is approved for:
- Patients with extensive-stage small cell lung cancer that has grown or spread. In such cases, Tecentriq may be used in combination with chemotherapies carboplatin (Paraplatin) and etoposide (Etopophos) as a first treatment.
Advanced triple-negative breast cancer
Tecentriq is approved for:
- Patients with triple-negative breast cancer (cancer that tests negative for estrogen hormone receptor, progesterone hormone receptor, and human epidermal growth factor receptor 2 [HER2]) that has grown or spread, cannot be removed by surgery, and tests positive for the PD-L1 molecule, and who have not received chemotherapy for their advanced disease. In such cases, Tecentriq may be used in combination with protein-bound paclitaxel (nab-paclitaxel; Abraxane).
Liver cancer
Tecentriq is approved for:
- Patients with liver cancer (hepatocellular carcinoma) that cannot be removed by surgery or has spread to other parts of the body, and who have not yet received systemic therapy (therapy by mouth or injection into the vein [i.v.]) for their disease. In such cases, Tecentriq is used in combination with bevacizumab (Avastin, Mvasi, Zirabev).
Limitations of use:
Age: The safety and efficacy of Tecentriq in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Tecentriq may impair fertility in women while receiving treatment. Tecentriq can cause harm to a fetus, and is not recommended for use during pregnancy. Pregnancy should be prevented for at least five months after the last dose of Tecentriq. The risks associated with Tecentriq during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for at least five months after the last dose of Tecentriq.
What type of immunotherapy is this?
How does this drug work?
Tecentriq is an antibody that attaches to a molecule called PD-L1, which is sometimes present on the surface of cancer cells or other cells within the tumor mass. PD-L1 interacts with a molecule called PD-1, which is present on the surface of T cells – the primary immune cells involved in killing cancer cells. In healthy tissues, the interaction between PD-L1 and PD-1 puts on a brake that keeps T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from doing their job – killing the cancer cells. When PD-L1 interacts with PD-1 on T cells, the T cells become inactive and do not attack the cancer cells. Tecentriq binds to the PD-L1 molecules on cancer cells and other cells within the tumor mass in such a way that it blocks the interaction between PD-1 and PD-L1, and allows the T cells to be active and attack the cancer cells.

How is this drug given to the patient?
Tecentriq is administered via a tube into a vein (intravenous infusion, or i.v.) over 60 minutes every two, three, or four weeks (depending on the dose) and does not require a hospital stay. If the first infusion is tolerated, all following infusions may be administered over 30 minutes.
What are the observed clinical results?
For:
Advanced bladder and urinary tract (urothelial cell) cancer
Early-stage non-small cell lung cancer (removed by surgery)**Advanced non-small cell lung cancer (previously untreated with chemotherapy)*Advanced non-small cell lung cancer (previously treated with chemotherapy)****Advanced small cell lung cancer*Advanced triple-negative breast cancer****Liver cancer**
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced bladder and urinary tract (urothelial cell) cancer
In a clinical trial, a group of 119 patients with advanced bladder cancer that had grown or spread AND:
were treated with Tecentriq. At a median follow-up of 17 months following treatment with Tecentriq:
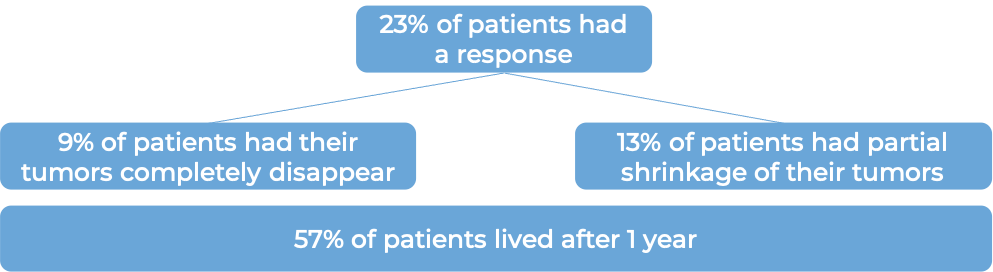
Among patients whose tumors tested positive for high levels of the PD-L1 molecule (32 patients):
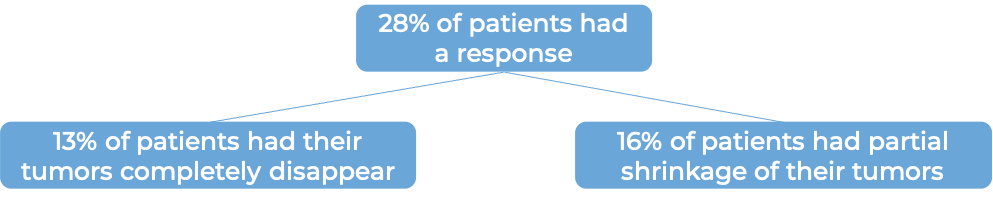
In the same clinical trial, a group of 310 patients with advanced bladder cancer that had grown or spread and who had been treated with chemotherapy containing platinum, but it did not work or or stopped working within 12 months were treated with Tecentriq. At a minimum follow-up of 24 months following treatment with Tecentriq:
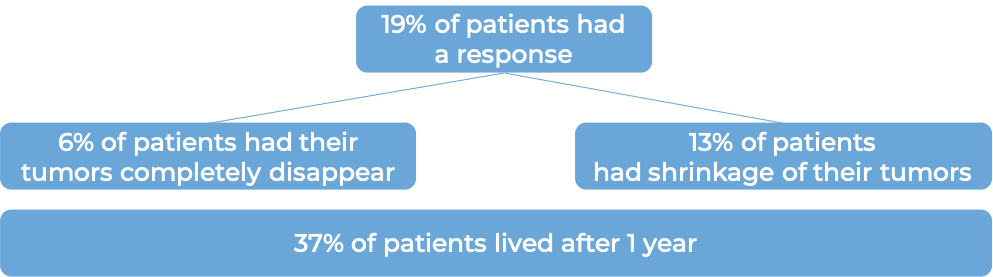
In another clinical trial, 931 patients with advanced urothelial cancer that had grown or spread, and who had been treated with chemotherapy containing platinum, but it did not work or stopped working, were treated with either Tecentriq or the investigator’s choice of chemotherapy (vinflumine, docetaxel, or paclitaxel). At a median follow-up of 34 months:
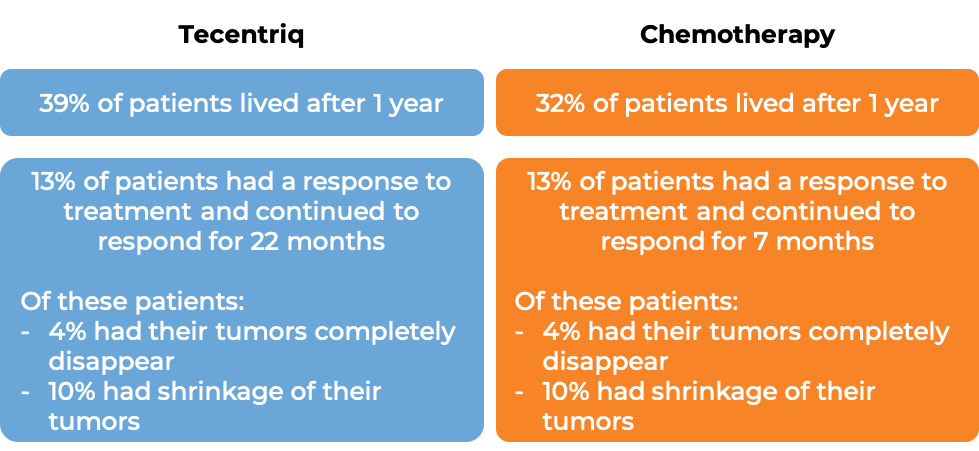
In another clinical trial, 719 patients with urothelial cancer that was locally advanced or had spread (metastatic) and who had not received treatment for their advanced disease were treated with either atezolizumab or chemotherapy. Those treated with atezolizumab survived for a median of 19 months while those treated with chemotherapy survived for a median of 10 months.
Early-stage non-small cell lung cancer (removed by surgery)
In a clinical trial, 1005 patients with non-small cell lung cancer were either treated with Tecentriq or best supportive care following complete surgical removal of all known disease and cisplatin-based chemotherapy. Among 209 patients whose tumor tested positive for high levels of the PD-L1 molecule, at a median follow-up of 32 months:

Advanced non-small cell lung cancer (previously untreated with chemotherapy)
In a clinical trial of patients with nonsquamous non-small cell lung cancer that had spread and who had not previously been treated with chemotherapy for their advanced disease, patients were treated with one of the following three treatment options:
- Treatment 1: Tecentriq in combination with paclitaxel (Taxol or Onxal) and carboplatin (Paraplatin)
- Treatment 2: Tecentriq in combination with bevacizumab (Avastin, Mvasi, Zirabev), paclitaxel (Taxol or Onxal), and carboplatin (Paraplatin)
- Treatment 3: bevacizumab (Avastin), paclitaxel (Taxol or Onxal), and carboplatin (Paraplatin)
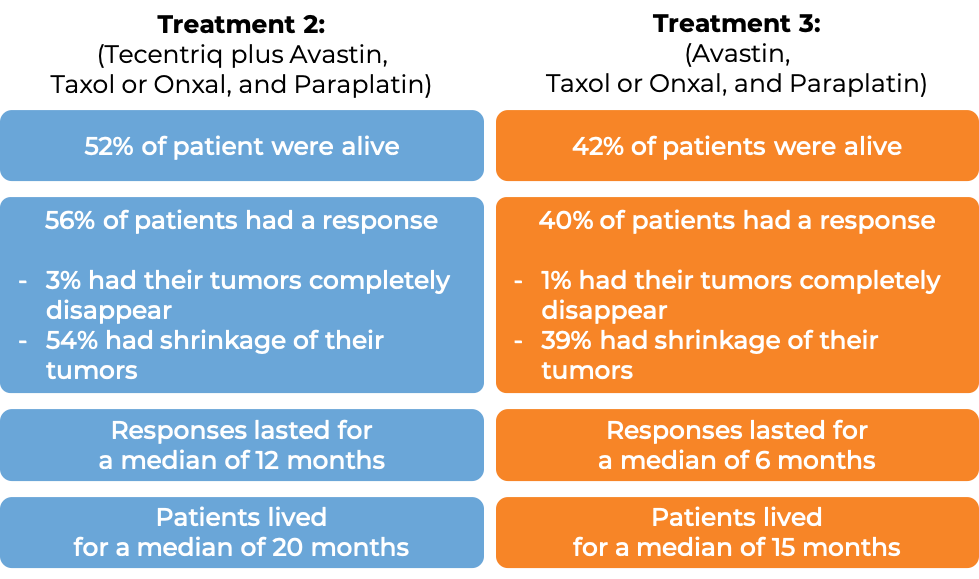
In the 1202 enrolled patients, at a median follow-up of 20 months, the following results were observed for patients receiving either Treatment 2 or 3:
(Results for patients receiving Treatment 1 did not significantly differ from the results observed with Treatment 3, and the combination used in the Treatment 1 arm did not receive approval.)
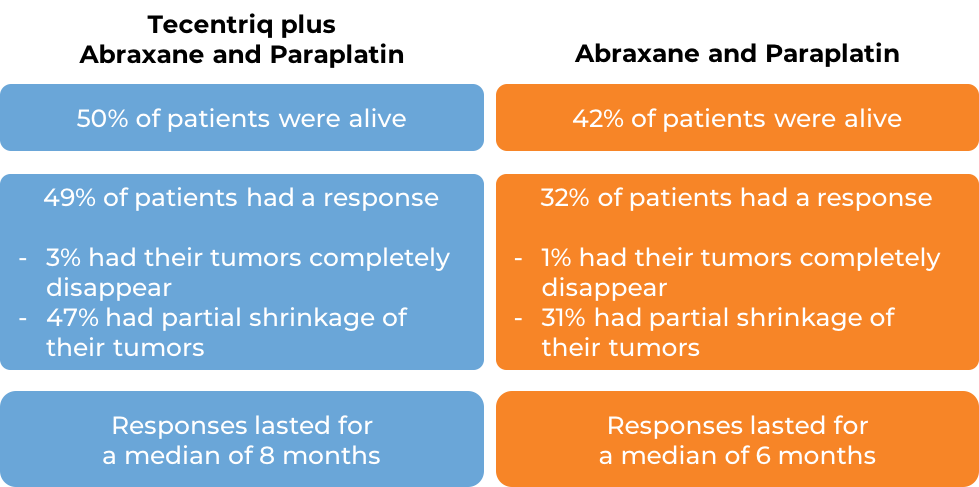
In another clinical trial of patients with nonsquamous non-small cell lung cancer that had spread and who had not previously been treated with chemotherapy for their advanced disease, patients were treated with either a combination of Tecentriq, protein-bound paclitaxel (nab-paclitaxel; Abraxane), and carboplatin (Paraplatin) or a combination of protein-bound paclitaxel (nab-paclitaxel; Abraxane) and carboplatin (Paraplatin). In the 679 of 724 enrolled patients whose cancer did not have an abnormal EGFR or ALK gene, at a median follow-up time of 19 months after treatment:
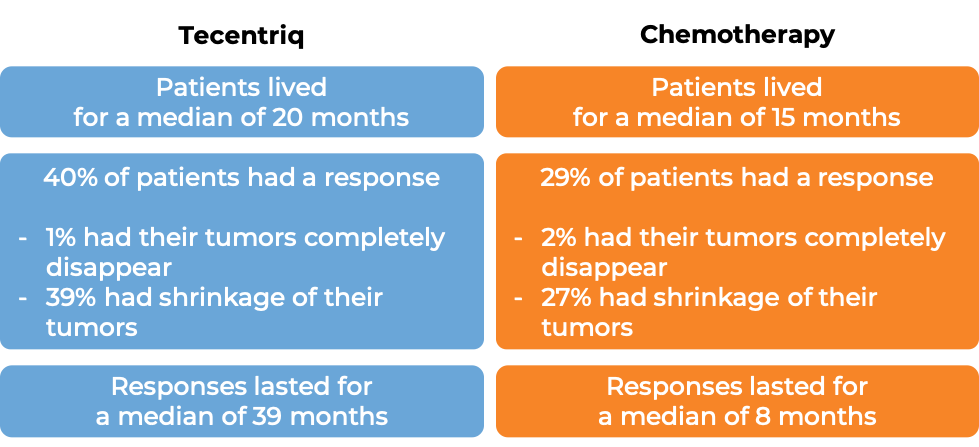
In another clinical trial, 572 patients with non-small cell lung cancer that had spread to other parts of the body, tested positive for the PD-L1 molecule or immune cells in the tumor bed, and had not yet been treated with chemotherapy for their advanced disease were either treated with Tecentriq or chemotherapy. At a median follow-up of 31 months:
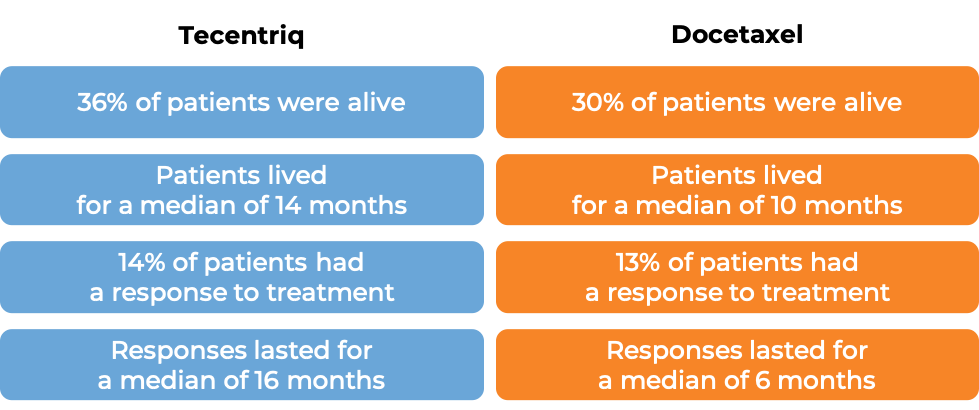
Advanced non-small cell lung cancer (previously treated with chemotherapy)
In a clinical trial of patients with non-small cell lung cancer that had grown or spread and who had already tried chemotherapy containing platinum, but it either did not work or stopped working, patients were treated with either Tecentriq or docetaxel (a chemotherapy). In the first 850 treated patients, at a median follow-up of 21 months following treatment:
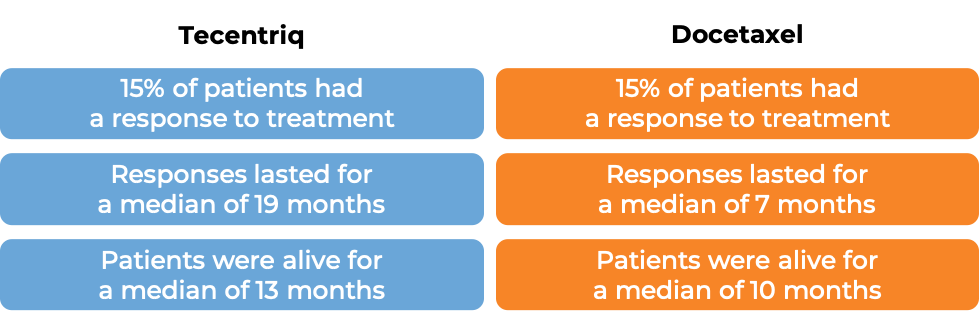
In another clinical trial of 287 patients with non-small cell lung cancer that had grown or spread and who had already tried chemotherapy containing platinum, but it either did not work or stopped working, patients were treated with either Tecentriq or docetaxel (a chemotherapy). At a median follow-up of 22 months following treatment:
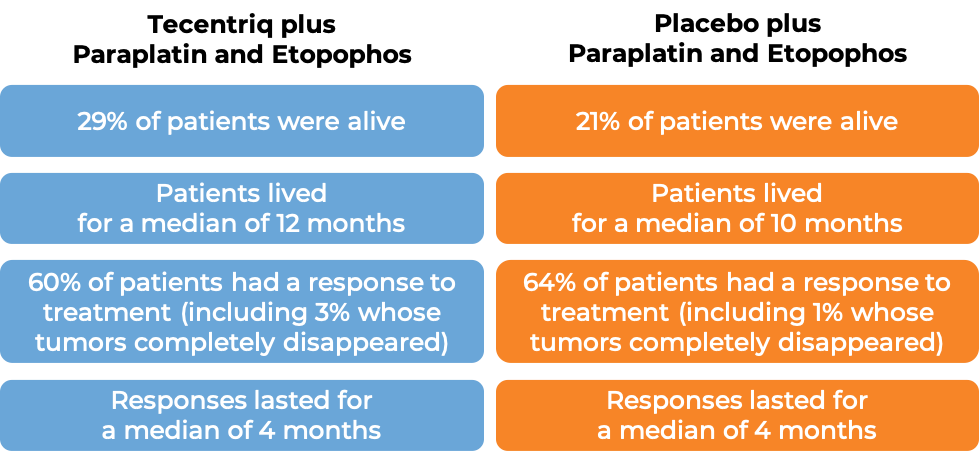
Advanced small cell lung cancer
In a clinical trial of 403 patients with extensive-stage small cell lung cancer that had grown or spread and who had not been treated with chemotherapy for their advanced disease, patients were treated with either Tecentriq in combination with the chemotherapies carboplatin (Paraplatin) and etoposide (Etopophos), or with a placebo in combination with carboplatin (Paraplatin) and etoposide (Etopophos). At a median follow-up of 14 months following treatment:
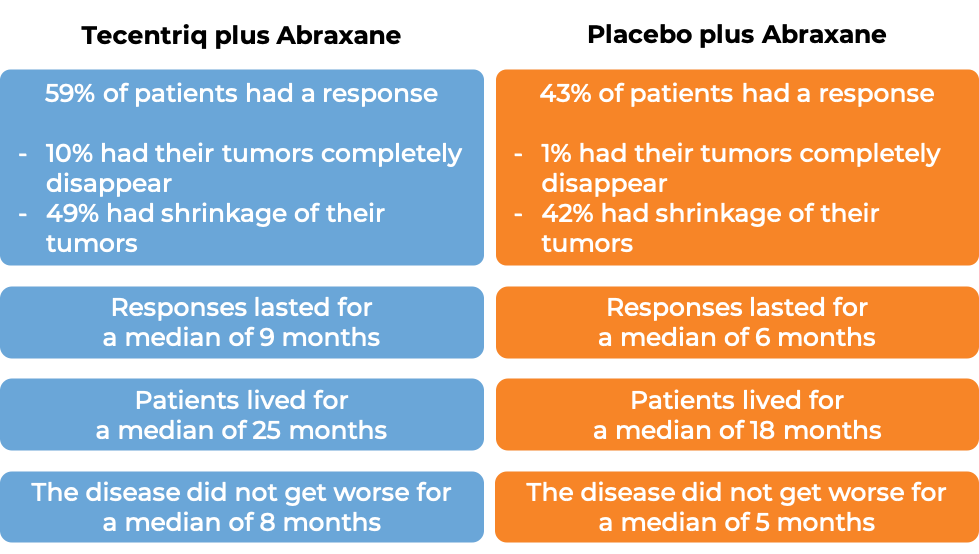
Advanced triple-negative breast cancer
In a clinical trial of 902 patients with triple-negative breast cancer that had grown or spread and could not be removed by surgery, and who had not been treated with chemotherapy for their advanced disease, patients were treated with either Tecentriq in combination with protein-bound paclitaxel (nab-paclitaxel; Abraxane) or with a placebo in combination with protein-bound paclitaxel (nab-paclitaxel; Abraxane). In 369 patients whose cancer tested positive for high levels of the PD-L1 molecule, at a median follow-up of 19 months following treatment:
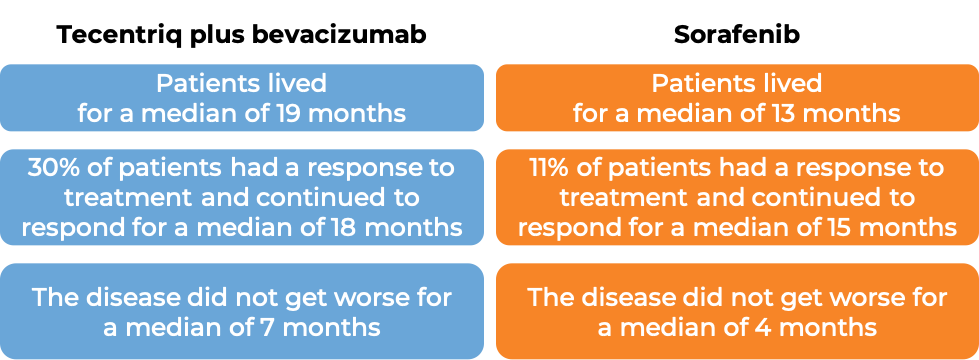
Liver cancer
In a clinical trial, 501 patients with liver cancer (hepatocellular carcinoma) that could not be removed by surgery or had spread to other parts of the body, and who had not received systemic therapy (therapy by mouth or injection into the vein [i.v.]) for their disease, were treated with either Tecentriq plus bevacizumab (Avastin, Mvasi, Zirabev) or sorafenib (Nexavar). At a median follow-up of 16 months:
What are the side effects?
The most common side effects of Tecentriq include fatigue, weakness, nausea, decreased appetite, cough, urinary tract infections, skin rash, itchy skin, back pain,and shortness of breath. When Tecentriq is used in combination with other drugs, other common side effects include diarrhea, constipation, vomiting, hair loss, headache, pain (in the muscles, bones, and joints), tingling or numbness in hands or feet, low red blood cell count (anemia), and low white blood cell count.
Tecentriq can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Tecentriq can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Tecentriq include inflammation of the lungs, liver, heart, kidney, or colon; skin reactions; and bodywide (systemic) inflammation in the form of hemophagocytic lymphohistiocytosis (HLH). Tecentriq can also cause issues related to the pericardium (the sac around the heart), including inflammation and fluid buildup. Additionally, problems can arise with the muscles, vision, and hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Tecentriq may cause Type 1 diabetes. Reactions related to the infusion may also occur.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Genentech
Approval
FDA and EMA
Links to drug websites
Last updated on July 16, 2024
























