How is the drug name pronounced?
Necitumumab: neh-sih-TOOM-oo-MAB
Portrazza: por-TRA-zuh
What cancer(s) does this drug treat?
Advanced squamous non-small cell lung cancer
Portrazza is approved for:
- Patients with squamous non-small cell lung cancer that has spread, and who have not received any other treatment for their advanced disease. In such cases, Portrazza is used in combination with the chemotherapies gemcitabine and cisplatin.
Limitations of Use
Age: The safety and efficacy of Portrazza have not been established in patients under 18 years of age.
Pregnancy/breastfeeding: Portrazza may cause harm to the fetus and is not recommended for use during pregnancy. Women are advised to use contraception during treatment with Portrazza and for at least 3 months after the last dose of Portrazza. The risks associated with Portrazza during breastfeeding are not known and cannot be ruled out. Due to the potential for serious side effects in the breastfed child, women are advised not to breastfeed during treatment with Portrazza and for at least 3 months after the last dose of Portrazza.
Exclusions: Portrazza should not be used to treat patients with non-squamous non-small cell lung cancer.
What type of immunotherapy is this?
- Cell growth inhibitor
How does this drug work?
Target:
- Epidermal growth factor receptor (EGFR)
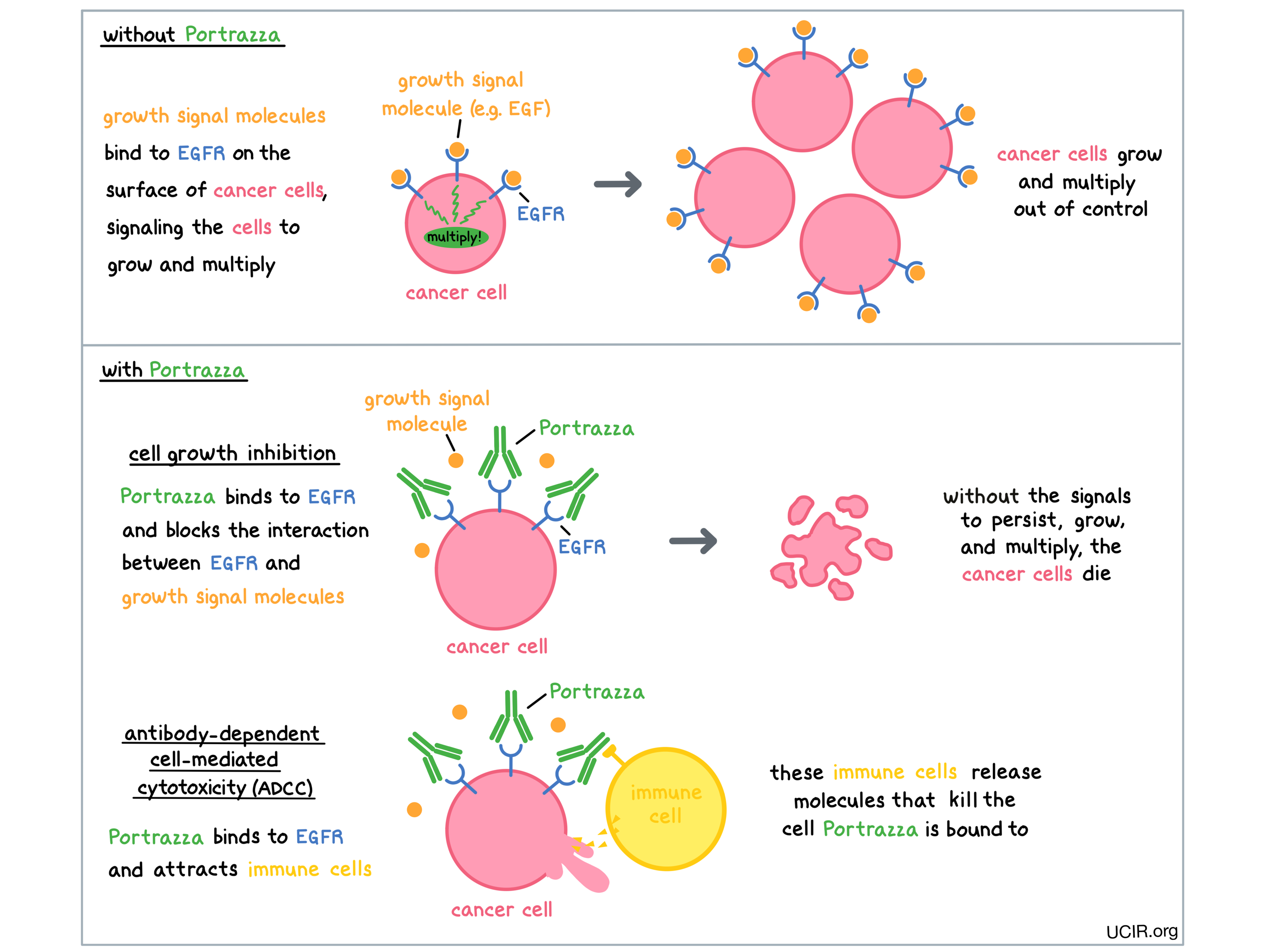
Portrazza is an antibody that was made in the laboratory and is designed to attach to a protein molecule called epidermal growth factor receptor (EGFR). EGFR is present on the surface of many normal cells (such as cells in the skin and hair follicles), and it is also present in much higher quantities on the surface of many types of cancer cells, including squamous non-small cell lung cancer. Higher-than-normal amounts of EGFR on cancer cells make these cells the main target of Portrazza.
Portrazza and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. The stem of Portrazza’s “Y” shape can attract immune cells or other parts of the immune system.
Portrazza works to kill cancer cells in at least two ways.
Cancer cell growth inhibition
When certain molecules (such as epidermal growth factor [EGF]) bind to EGFR on the surface of cells, the cells receive signals to ensure their survival and encourage the cells to grow and multiply. Higher-than-normal amounts of EGFR allow cancer cells to grow and multiply out of control. By binding to EGFR, Portrazza blocks these signals and prevents the cells from persisting and multiplying.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to EGFR on the surface of cancer cells, the “stem” of Portrazza can also attract and bind immune cells (like NK cells). This allows Portrazza to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell Portrazza is bound to.
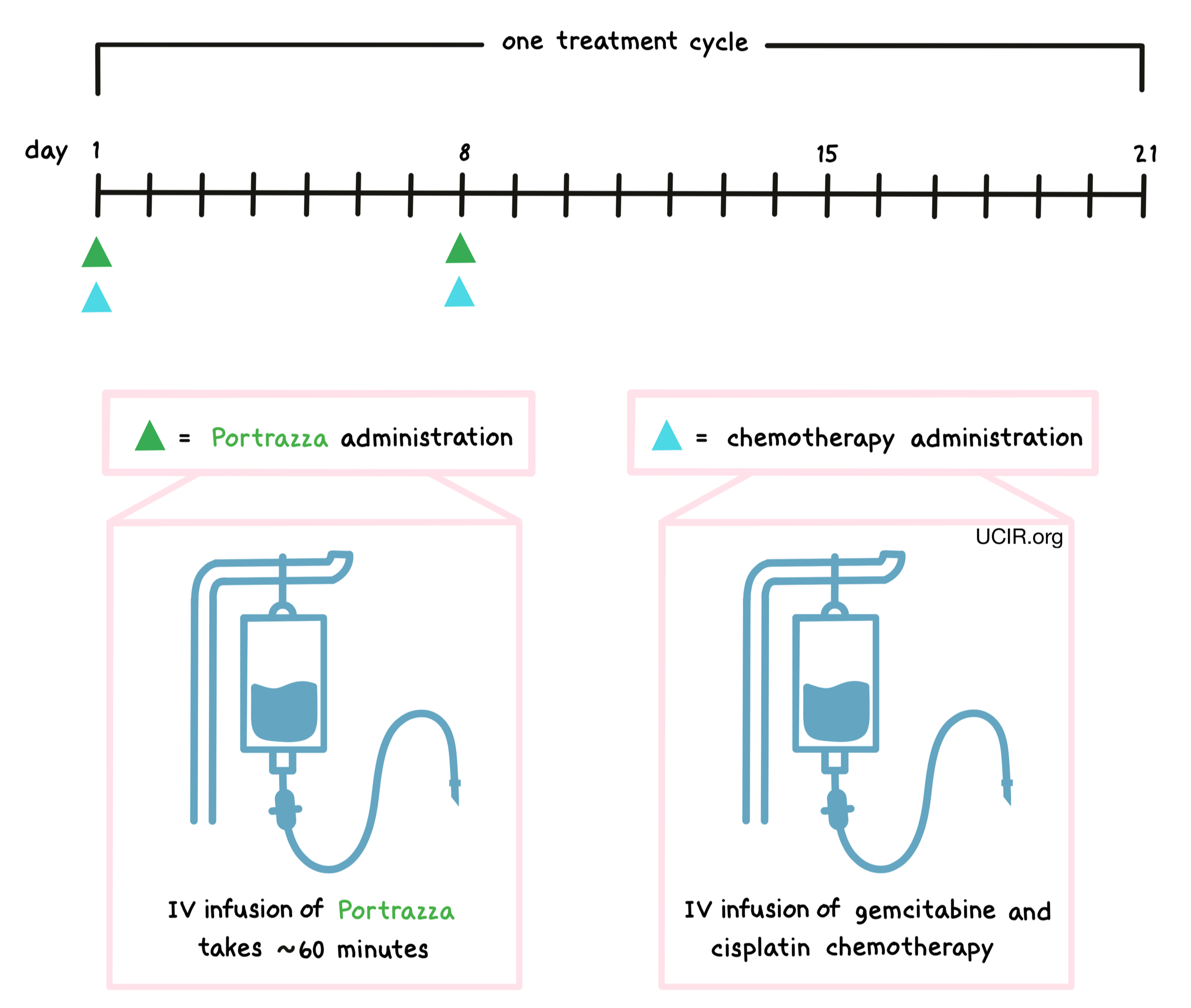
How is this drug given to the patient?
Portrazza is administered via a tube in the vein (intravenous infusion, or I.V.) over 60 minutes prior to treatment with an infusion of gemcitabine and cisplatin chemotherapy on days 1 and 8. This is then followed by a 13-day break. Each 3-week period is one “treatment cycle”. The healthcare provider decides how many treatment cycles the patient receives. Administration of Portrazza does not require a hospital stay.
During administration of Portrazza, patients should be closely monitored for reactions to the infusion. If symptoms of an infusion-related reaction occur, the infusion may be interrupted or slowed, depending on the severity of the reaction. Patients who experience an infusion-related reaction are treated with diphenhydramine, acetaminophen, and dexamethasone shortly before receiving their next dose of Portrazza.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced squamous non-small cell lung cancer
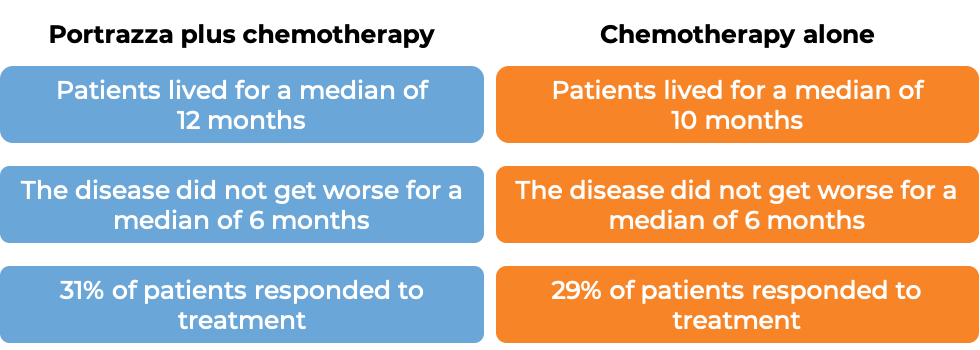
In a clinical trial, 1093 patients with squamous non-small cell lung cancer that had spread to other parts of the body, and who had not received any other treatment for their advanced disease were treated with Portrazza plus gemcitabine and cisplatin chemotherapy or gemcitabine and cisplatin chemotherapy alone. Patients received a maximum of 6 treatment cycles, and patients whose disease improved or did not get worse continued treatment with Portrazza without chemotherapy. 51% of patients initially receiving Portrazza plus chemotherapy went on to receive Portrazza alone. At a median follow-up of 25 months:
What are the potential side effects?
The most common side effects of Portrazza include rash, diarrhea, vomiting, skin inflammation that looks like acne, itching, dry skin, sore mouth and lips, weight loss, coughing up blood, headache, reactions related to the infusion, and low levels of electrolytes (including magnesium, calcium, phosphate, and potassium).
Portrazza can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Portrazza include severe skin reactions (which could lead to severe infections), severely low levels of electrolytes, problems with the heart (including heart attack), and blood clots in veins or arteries.
Low levels of magnesium in the body (hypomagnesemia) Symptoms of hypomagnesemia include involuntary twitching, especially of the facial muscles, physical weakness or an unexplained feeling of exhaustion, tremors, constipation, nausea or vomiting, muscle cramps, personality changes, fluttering sensations in the chest, or chest pain. If left unaddressed, hypomagnesemia can lead to severe heart problems, such as prolonged uneven heartbeats.
Infusion-related reactions Infusion-related reactions are side effects related to receiving an infusion. Most infusion-related reactions typically occur within 24 hours of the first or second infusion. Symptoms of an infusion-related reaction include hives or rash, itchiness, swelling of the tongue, lips, face, or throat, coughing, shortness of breath, wheezing, difficulty breathing, weakness, dizziness, feeling as if your heart is fluttering or racing, and chest pain.
Blood clots of veins or arteries (venous and arterial thromboembolic events) Venous and arterial thromboembolic events are caused by blood clots, either in deep veins or in arteries. Symptoms of these events may vary depending on the affected body part, but can include sudden pain in the arms, legs, or other parts of the body. This pain is often similar in sensation to a muscle cramp. Usually the pain is accompanied by the affected body part becoming visibly swollen, discolored, or warm to the touch. These events are serious, and may lead to heart attacks or other complications if left unaddressed. Blood clots that occur in the arteries of the lungs can present with rapid or difficult breathing, dizziness, chest pain that typically worsens when breathing in, rapid heart rate, coughing up blood, or loss of consciousness.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer
Eli Lilly and Company
Approval
FDA
Links to drug websites
Other references
Last updated on January 16, 2021


