Neoantigen-Based Therapy
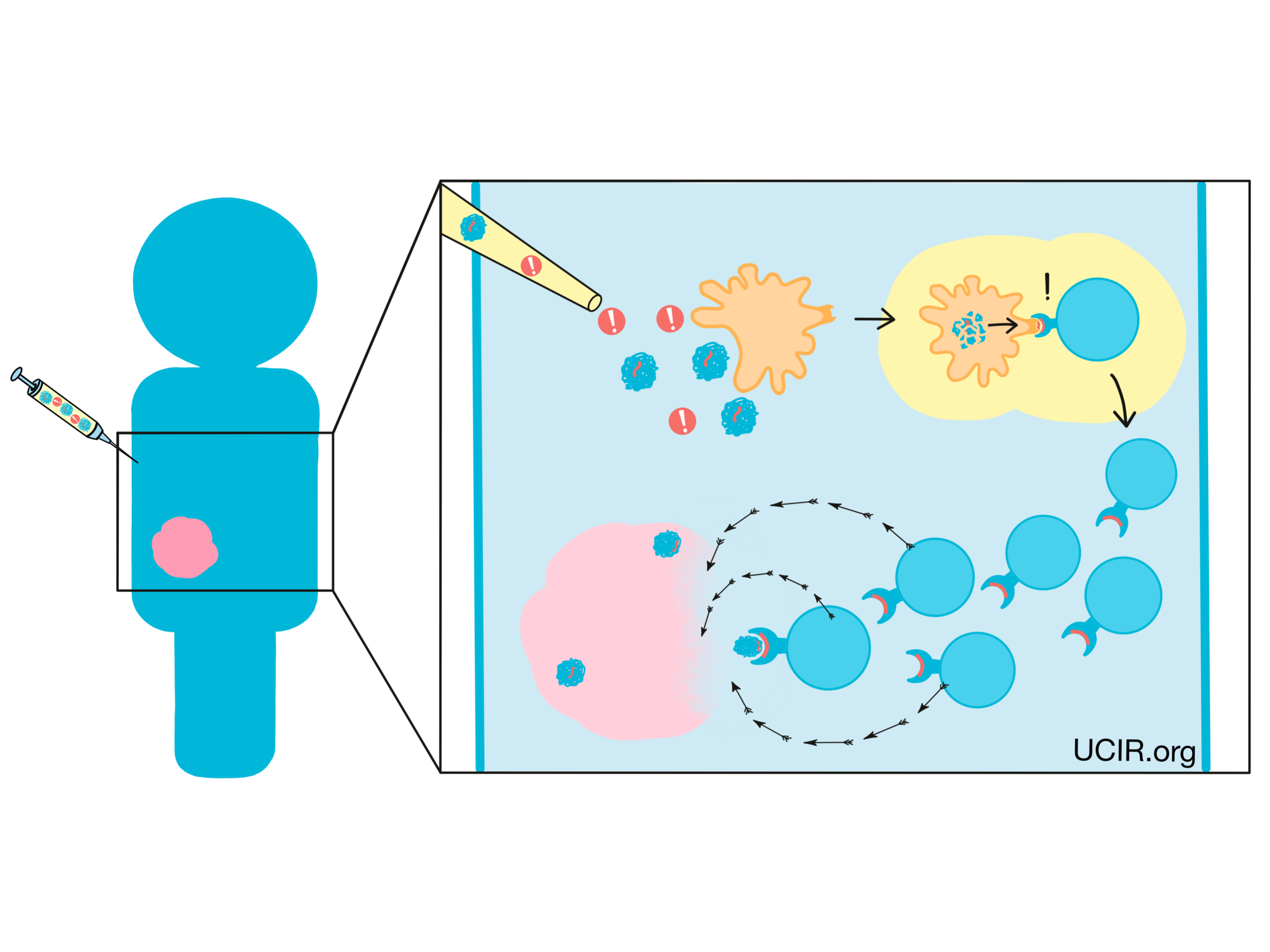
Background
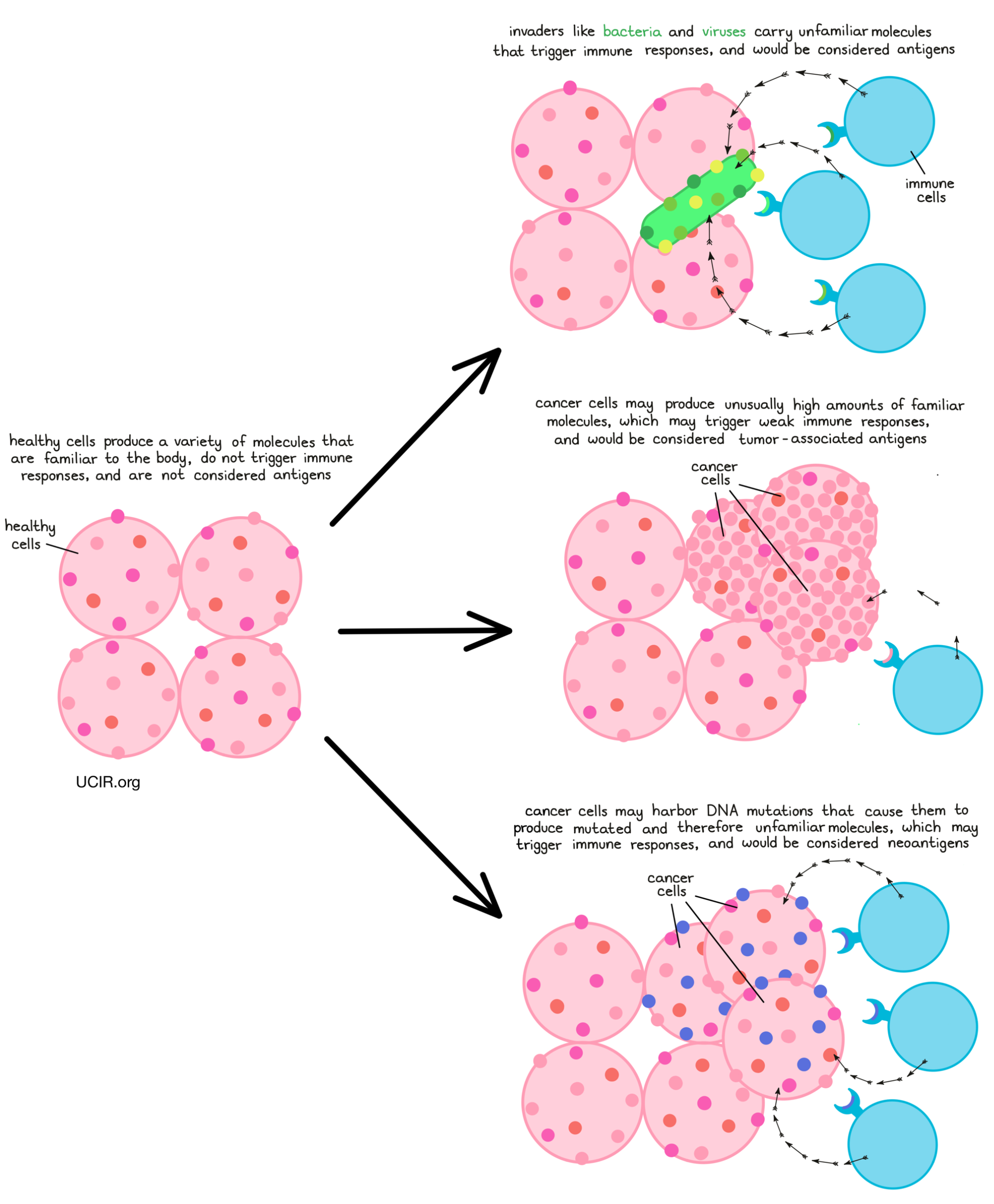
The main purpose of the immune system is to recognize cells that have been infected by a virus or bacteria, or cells that are behaving abnormally, and eliminate them from the body. To do this, the immune system patrols the body in search of antigens – protein molecules that are not found in the normal healthy body. Antigens are often unfamiliar molecules, like those introduced by invading viruses or bacteria. In some cases though, antigens may include familiar molecules that are unusually abundant, molecules that turn up in tissues where they are not usually found, or molecules that are common during early phases of development, but should not crop up later in life. When the immune system identifies these unfamiliar molecules, an immune attack may be triggered.
Cancer cells can also often carry unfamiliar molecules that can trigger immune responses. Cancer arises when normal cells in the body begin to multiply out of control due to mutations in their DNA. A cell’s DNA is the instruction manual for exactly how to build and operate the cell, and mutations are essentially typos in the DNA. While most mutations are fairly harmless and easy to ignore, some can alter the meaning of what’s written in the manual.
DNA provides the ‘code’ for how and when to make proteins. Proteins are large molecules that are responsible for building and maintaining the structure of a cell and for carrying out all of a cell’s operations. Proteins are formed when smaller molecules called amino acids are strung together in a particular order, with the order being critical to how that protein works.
What are neoantigens?
Mutations, changes in the DNA code, may lead a cell to produce excess amounts of a particular protein, produce a protein in the wrong type of cell or tissue, or produce a protein at the wrong time. When this occurs in cancer, that protein may be recognized by the immune system, making it a tumor-associated antigen. In other cases, a mutation may cause a cell to string together different amino acids, ultimately forming a protein that is entirely new and looks unfamiliar to the immune system, including T cells. These new and unfamiliar proteins that arise due to mutations are called neoantigens.
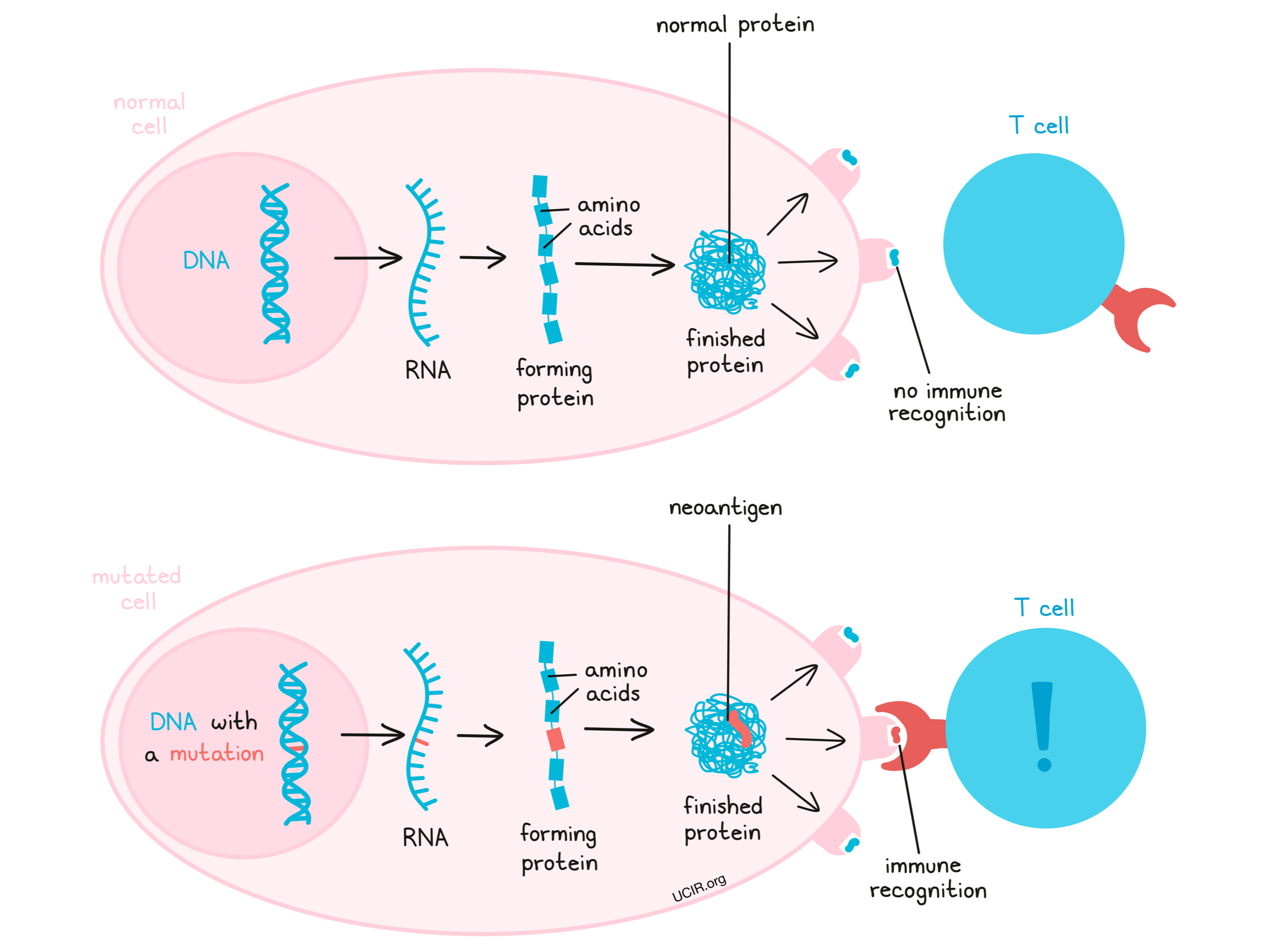
Both tumor-associated antigens and neoantigens can serve as good targets for immunotherapy, however, neoantigens may be the stronger choice. Neoantigens are more likely than tumor-associated antigens to trigger a strong immune response, as they are entirely unfamiliar to the body – like the proteins of a virus or bacteria are – and easily stand out as molecules that do not belong. Further, while tumor-associated antigens may be found at low levels elsewhere in the body, neoantigens are completely unique to the cancer cells, so targeting neoantigens for immunotherapy comes with a lower risk of accidentally harming healthy cells along the way. For these two reasons – triggering stronger immune responses and being present only in cancer cells – neoantigens have become a preferred target for immunotherapy.
Personal neoantigen-targeted immunotherapy
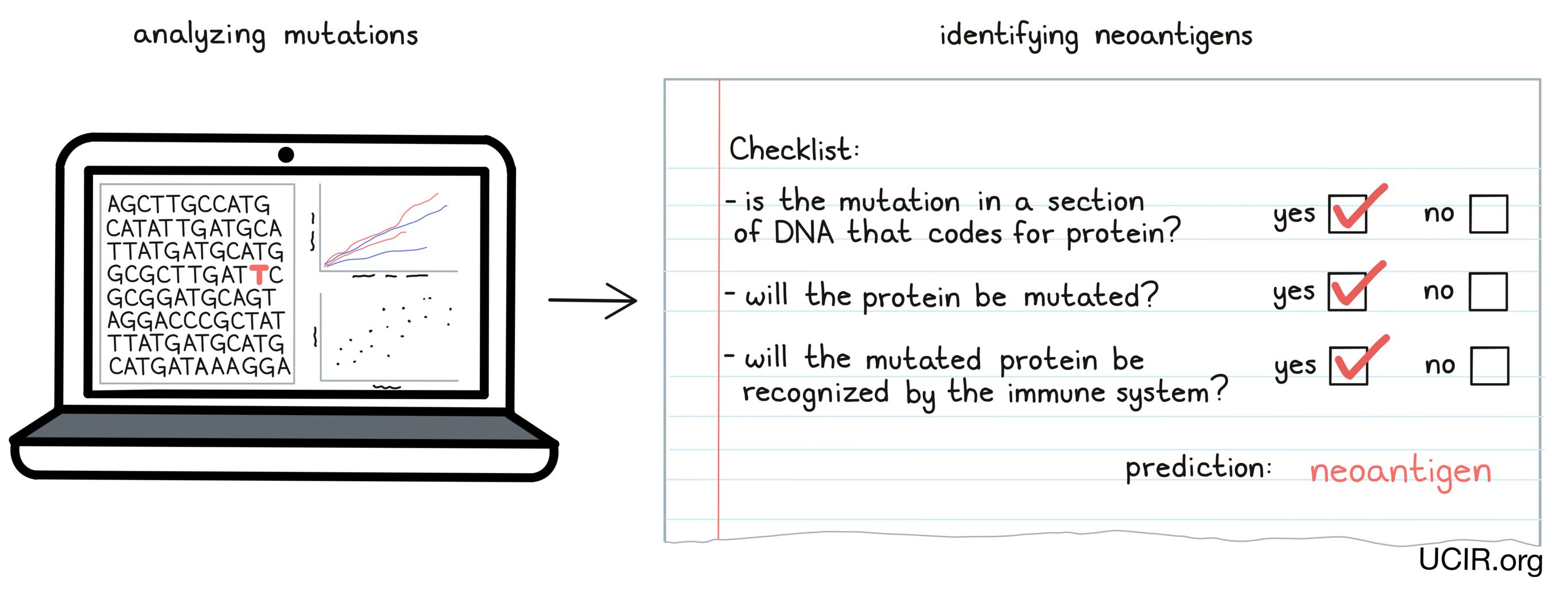
When DNA mutates and a normal cell becomes a cancer cell, the vast majority of the mutations will be unique to the cancer in an individual, and certain immunotherapies can take advantage of this. The first step to targeting personal neoantigens is identifying the DNA mutations in the cancer cells. To do this, doctors must first obtain a sample of a patient’s cancerous cells, either through a tumor biopsy or surgical resection, along with a sample of healthy cells – usually a blood sample, but sometimes a cheek swab. Once these samples have been obtained, the DNA can be sequenced and compared, and any mutations in the cancer can be identified.
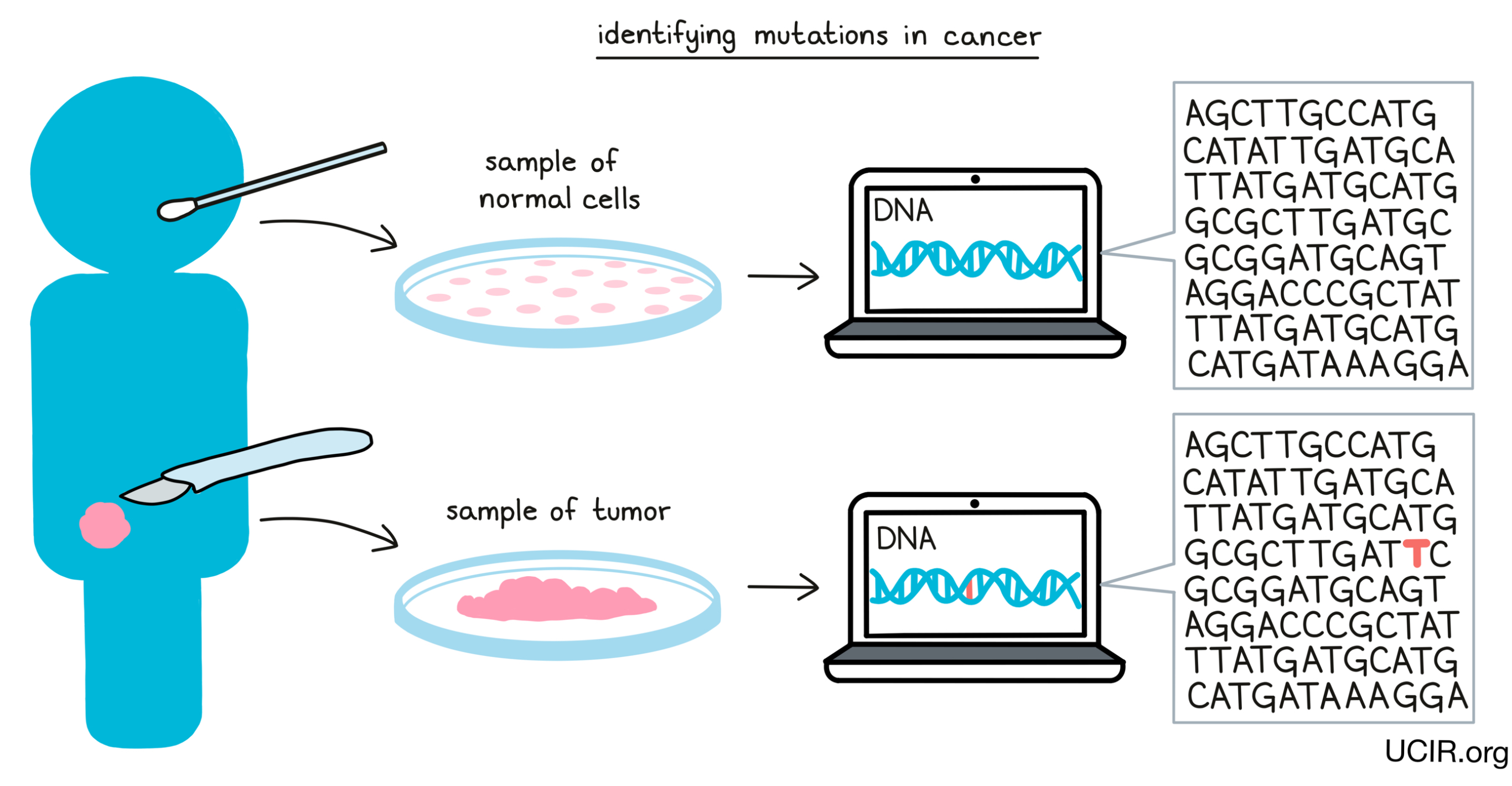
Once mutations in cancer have been identified, researchers can analyze them and predict whether individual mutations are likely to result in the production of neoantigens, and whether those neoantigens are likely to be “seen” and recognized by immune cells. Only a small number of all the mutations that are present in cancer cells result in neoantigen targets that can be used in treatment, and finding these “needles in the haystack” is an area of intensive research.
Once this “bioinformatic analysis” of the patient’s DNA sequence is complete, a list of neoantigens to target for each individual patient can be prepared. This list is different for each patient.
Targeting shared neoantigens
Most mutations in cancer are unique to an individual patient, however, a few mutations are commonly shared between some patients. Most often, these shared mutations are driver mutations that disrupt the function of certain key proteins (like KRAS, BRAF, and p53) and drive cells to become cancerous. Since these same mutations are seen in a high portion of cancers, it might be possible to target the shared neoantigens that arise when these mutations occur. This strategy could bypass the need to personalize neoantigen-targeting treatments, making them faster and more affordable by mass production. Although this approach sounds attractive, the hunt is still on to identify neoantigens that are shared between multiple patients and are good targets for the immune system.
How are neoantigens used in cancer immunotherapy?
If a patient’s cancer contains mutations that are likely to result in strong neoantigens, researchers may follow one of two immunotherapeutic approaches to target them: cancer vaccine therapy or T cell therapy. Cancer vaccines are used to “train” T cells that are already present in the patient’s body, while T cell therapy involves taking some T cells out of a patient, “training” them in a laboratory, and then delivering them back into the patient.
Neoantigen-targeted cancer vaccines
Vaccines have been used for centuries to prevent the spread of infectious diseases. They work by delivering the antigens of a virus or bacteria to a person to stimulate an immune response that protects the person from future infection.
Neoantigen-targeted cancer vaccines use the neoantigens identified in an individual patient to create or strengthen an immune response to those neoantigens. A patient with cancer may already have a natural immune response to some of these neoantigens, but a vaccine can give that natural immunity a massive boost. In some cases, the vaccine will create new responses to neoantigens.
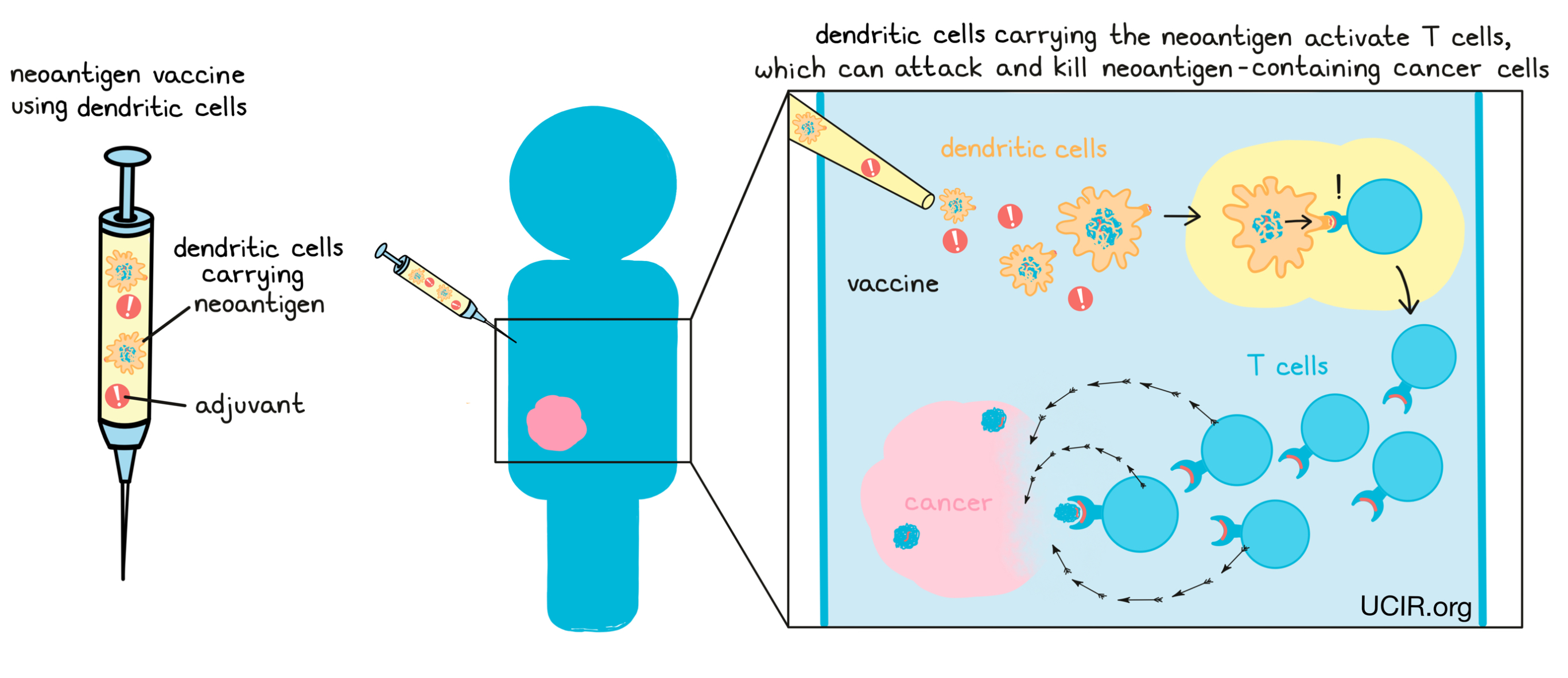
If a neoantigen vaccine is successful, antigen-presenting cells like dendritic cells will pick up the neoantigens from the vaccine and use them to activate T cells. Activated T cells then multiply, forming an immune army that can attack and kill any cells that contain the neoantigens, which would lead them straight to the cancer.
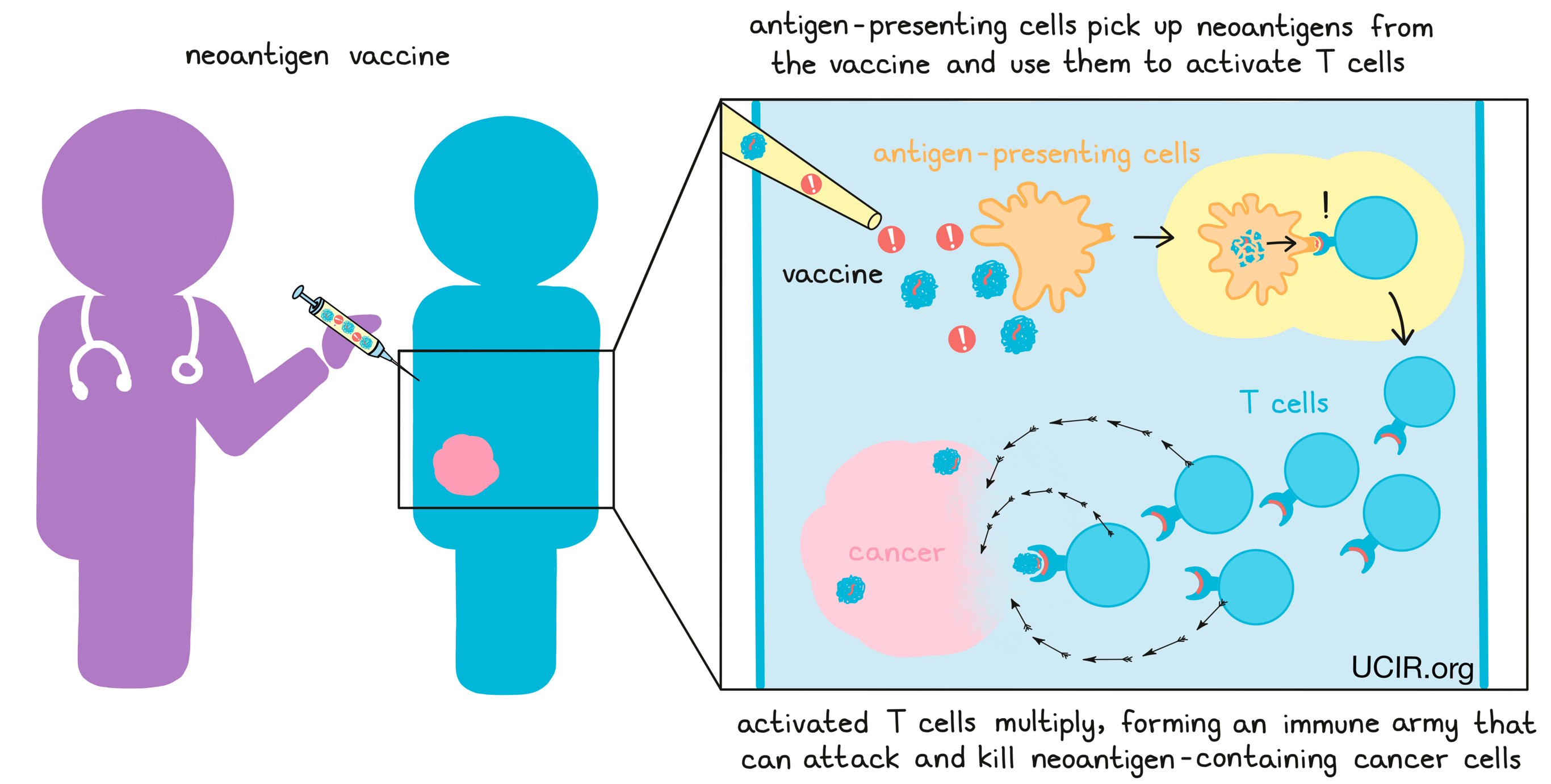
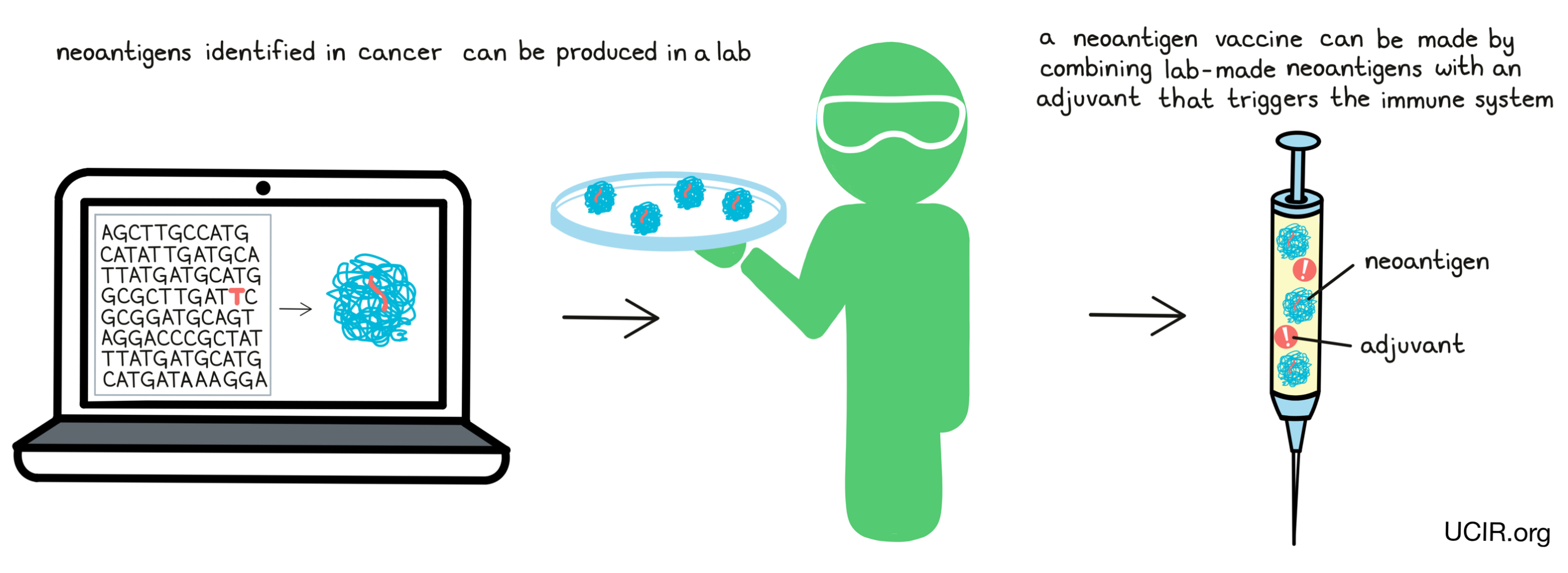
In some cases, neoantigen vaccines consist of lab-made versions of the neoantigens, along with an adjuvant – a substance that provides “danger signals” and alerts the immune system of the threat.
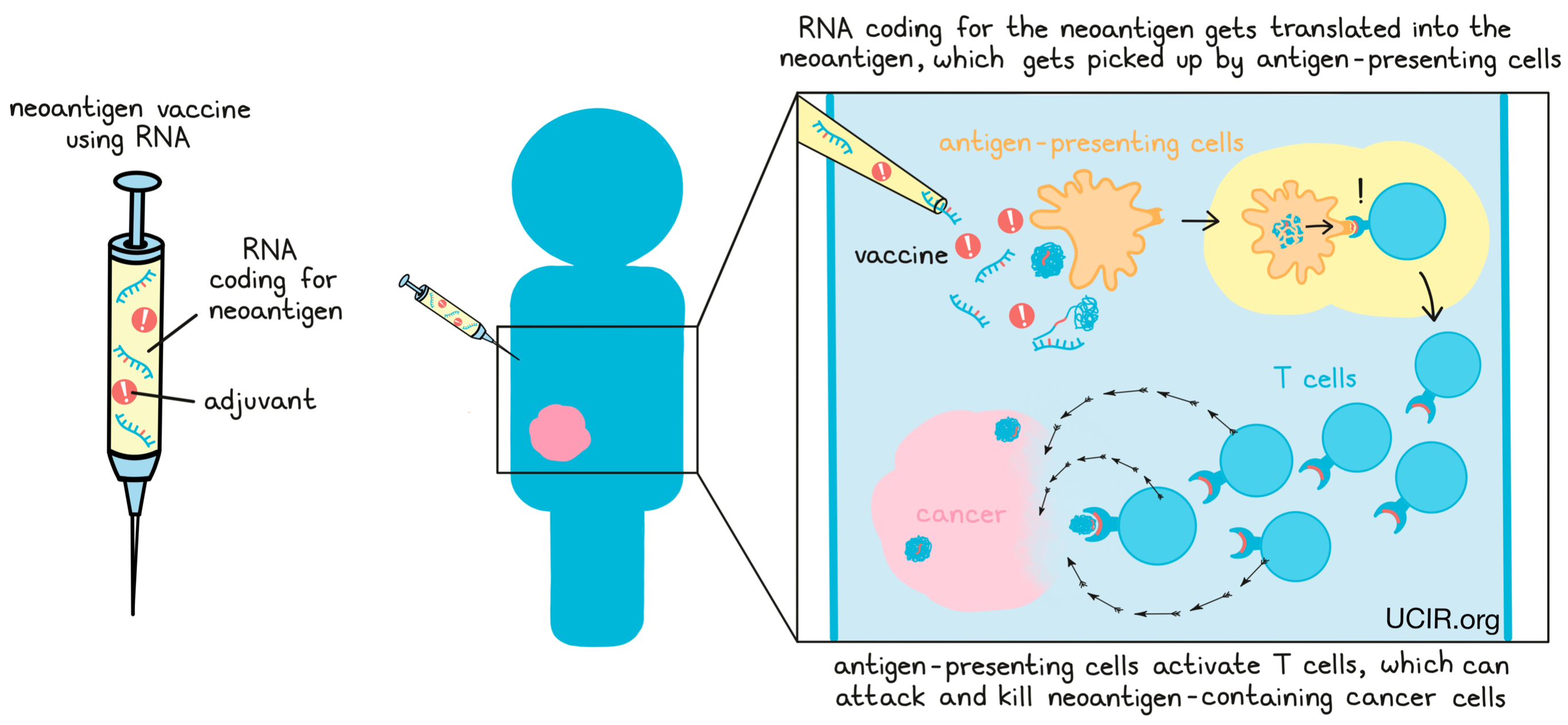
As an alternative to using lab-made neoantigens, some vaccines utilize RNA, or DNA that contains the code for the neoantigens. In these cases, the vaccine instructs cells in the body to translate the RNA or DNA code and make the neoantigens, which can then activate an immune response.
Another alternative is to take dendritic cells from the patient and grow them outside of the body in the presence of neoantigens. The dendritic cells pick up the neoantigens and are then injected back into the patient where they can activate the patient’s T cells. This strategy bypasses the need to rely on the dendritic cells within a patient’s body to pick up the neoantigens.
Finally, cancer vaccines have been developed that do not rely on identifying neoantigens in the cancer cell to prepare the vaccine, but instead use the cancer cells themselves (or the proteins or the RNA from those cells), as the vaccine. The idea is that all of the neoantigens (and also all of the tumor-associated antigens), will be present in these cells, whether researchers have identified them or not. To make these vaccines, cancer cells that have been removed from the patient are killed; in some cases, they are broken down into parts. The dead cancer cells themselves or components of the dead cancer cells are then combined with adjuvants, forming a vaccine that stimulates the immune system to respond to cancer antigens, including both tumor-associated antigens and neoantigens.
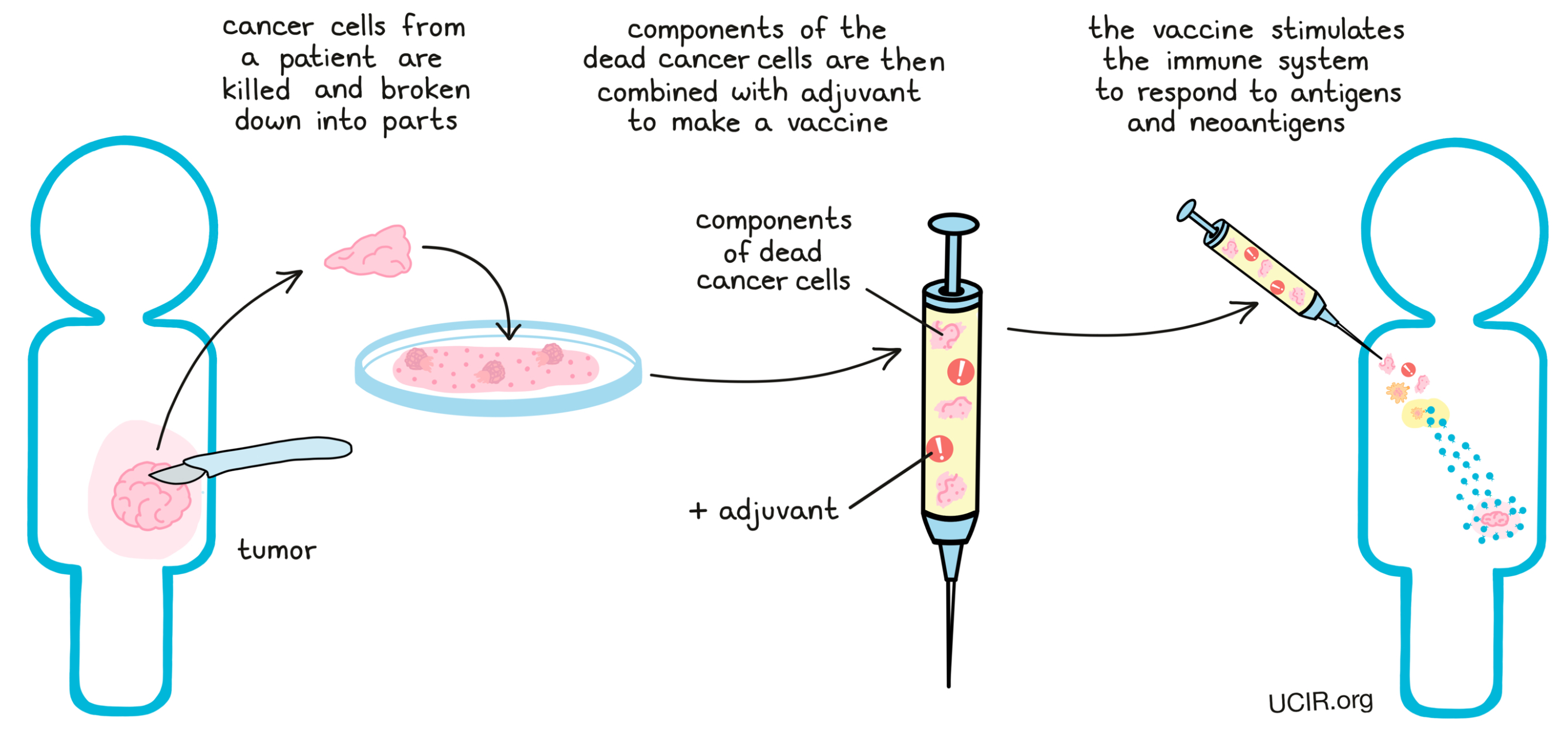
While all these cancer vaccine strategies differ slightly, they all ultimately serve to boost the immune system’s response to neoantigens in the cancer by training the immune system to recognize those neoantigens as a threat.
Neoantigen-targeted T cell therapy
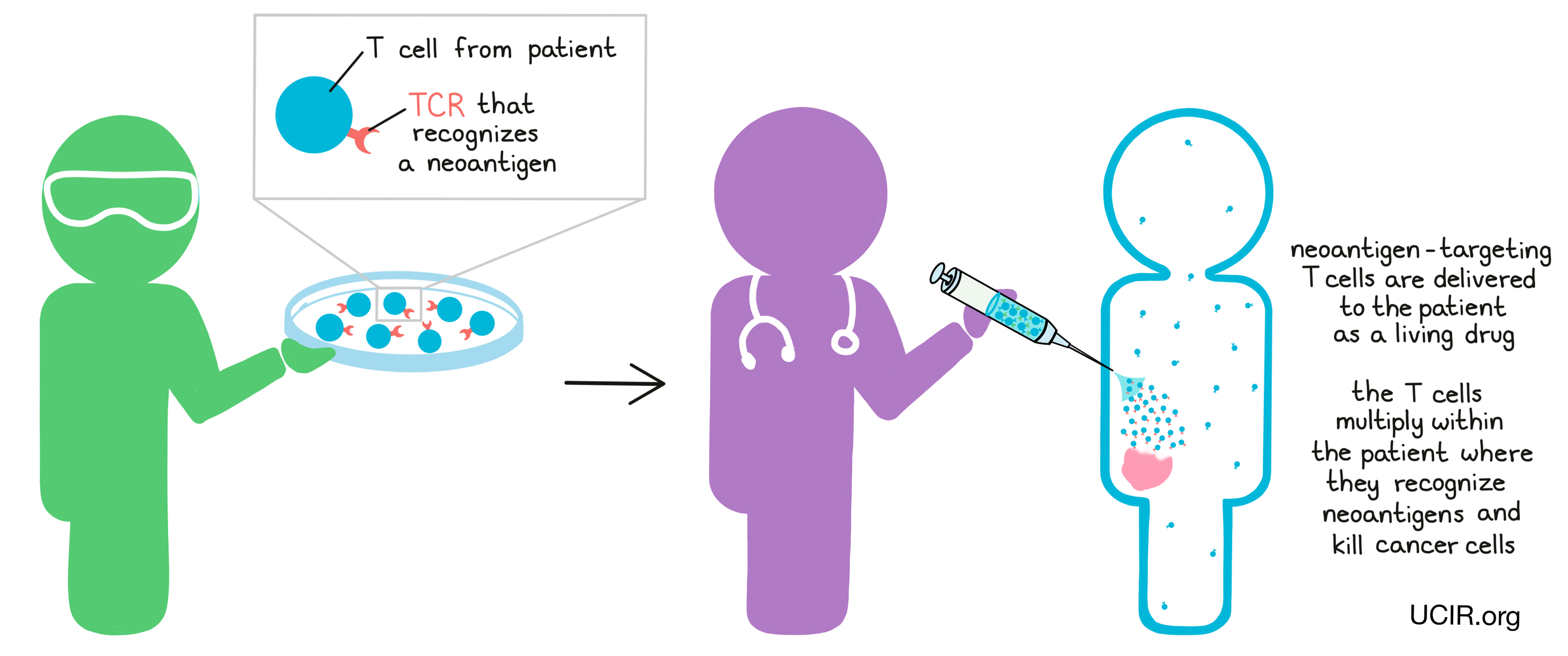
Neoantigen-targeted T cell therapy is a type of T cell therapy in which T cells are collected from the patient – either from the blood or directly from a tumor sample. From these T cells, naturally occuring T cells that have T cell receptors (TCRs) that recognize neoantigens can be isolated. Then, in the lab, these cells can be strengthened and increased in numbers before being given back to the patient as a living drug. The T cells then continue to multiply within the patient, where they recognize neoantigens and kill cancer cells.
In other cases, researchers may use T cell receptor (TCR) engineering to force T cells to target neoantigens. For TCR engineering, T cells are collected from a patient’s blood and a gene is added to code for a T cell receptor that recognizes a neoantigen that is known to be present in the patient’s cancer. These TCR-engineered T cells can then be grown and multiplied in the lab to form a massive T cell army that can be delivered back to the patient. When the engineered T cells are delivered back into the patient, they hunt down and attack any cells containing the neoantigens, leading them directly to cancer cells.
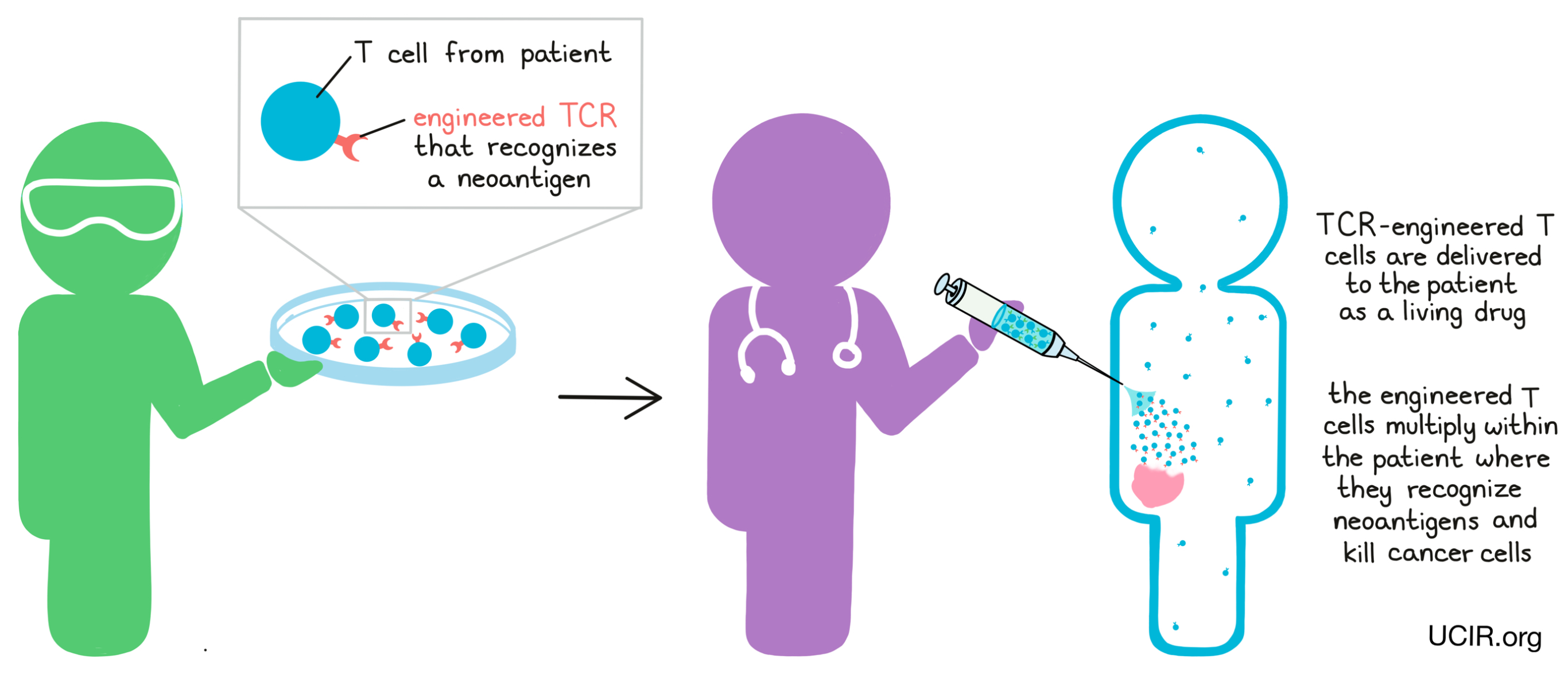
T cell receptor engineering is currently focused on shared neoantigens, though researchers hope to one day apply it to personal neoantigens.
Indirect targeting of neoantigens
Immunotherapies that broadly enhance immune responses likely work in part by enhancing immune responses to neoantigens. For example, checkpoint blockade therapy may enhance pre-existing, but weak T cell responses to neoantigens, while oncolytic virus therapy may force hidden neoantigens out into the open. Patients with a high number of mutations in their cancer tend to respond better to such therapies. The identification of numerous neoantigens, or of particularly strong neoantigens, could help researchers and doctors determine which patients are most likely to benefit from certain treatments. A very active field of research is combining neoantigen cancer vaccines with checkpoint blockade therapy.
Neoantigen targeting in the clinic
Currently, no strategies that specifically target neoantigens have been clinically approved, however, many neoantigen-targeting cancer vaccines or T cell therapies are currently being developed and tested in clinical trials.
Toxicities, Side Effects, and Shortcomings
Typically, cancer vaccines demonstrate significantly fewer and less severe side effects than other therapies such as chemotherapy, radiation therapy, or immune checkpoint blockade therapies.
Targeting neoantigens almost always requires that patients’ tumors are accessible for biopsy or surgical resection; if tumor is not accessible, or in rare cases when a large enough sample cannot be taken out, then researchers may not have access to the tumor DNA that they need to identify neoantigens.
Identification of neoantigens involves DNA analysis and developing personalized treatments, which can be time-consuming and expensive. Finally, not all cancers contain neoantigens (or enough neoantigens) that are suitable to target.
Which patients will benefit from neoantigen targeting?
Successful neoantigen targeting depends on the individual mutations that are present in a patient’s cancer. Cancers with a high mutation burden, mismatch-repair deficiency, or microsatellite instability may offer more potential neoantigens to target.
What’s next for neoantigen targeting research?
First and foremost, neoantigen-targeted therapies are currently being tested in multiple clinical trials and will hopefully be moving towards clinical approval. Research is being done in order to improve the identification of mutations in the DNA of small tumor samples, enhance the ability to predict whether DNA mutations will lead to neoantigens that can activate an effective immune attack, and reduce the cost and the time it takes to manufacture personalized neoantigen-targeted immunotherapies.
Further information
- Personal Neoantigen Cancer Vaccines: A Road Not Fully Paved (Review article) Fritsch EF, Burkhardt, UE, Hacohen N, Wu CJ. Cancer Immunology Research (2020)
- Neoantigens in cancer immunotherapy (Review article) Schumacher TN, Schreiber RD. Science (2015)
- Adoptive cell transfer as personalized immunotherapy for human cancer (Review article) Rosenberg SA, Restifo NP. Science (2015).


