How is this drug name pronounced?
Trastuzumab and Hyaluronidase: tras-TOOZ-ue-mab & HYE-al-ure-ON-i-dase Herceptin Hylecta: her-SEP-tin hy-LEK-tuh
What cancer(s) does this drug treat?
Early breast cancer
Herceptin Hylecta is approved for:
-
Patients with breast cancer that has spread within the breast or to the lymph nodes under the arms, but not to other parts of the body, and tests positive for high amounts of the HER2 molecule. In such cases, Herceptin Hylecta is used after surgical removal of all known disease. It may be used:
- Together with doxorubicin, cyclophosphamide, and either paclitaxel or docetaxel chemotherapy, OR
- together with docetaxel and carboplatin chemotherapy, OR
- by itself, for patients who have previously received an anthracycline-based chemotherapy treatment.
Advanced breast cancer
Herceptin Hylecta is approved for:
-
Patients with advanced breast cancer that tests positive for high amounts of the HER2 molecule and has spread to other parts of the body beyond the breast and lymph nodes. In such cases, Herceptin Hylecta is used:
- together with paclitaxel, for patients who have not yet received chemotherapy for their advanced disease, OR
- by itself, for patients who have previously received chemotherapy treatment for their advanced disease.
Limitations of Use
Age: The safety and efficacy of Herceptin Hylecta in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Treatment with Herceptin Hylecta can cause serious side effects and death to a fetus. Patients should use effective contraception to prevent pregnancy during treatment with Herceptin Hylecta and for at least seven months after the last dose. Herceptin Hylecta may cause adverse reactions in a breastfed child. Due to the potential for reactions in the breastfed child during treatment with Herceptin Hylecta and for at least seven months after the last dose of Herceptin Hylecta, the benefits of breastfeeding and the mother’s need for treatment should be weighed accordingly before moving forward with treatment with Herceptin Hylecta.
Drug interactions: Treatment with anthracycline-based chemotherapy (e.g., doxorubicin) should be avoided for up to 7 months after stopping Herceptin Hylecta. Patients who receive anthracycline-based chemotherapy less than 7 months after stopping Herceptin Hylecta may be at an increased risk for developing heart problems.
What type of immunotherapy is this?
- Cell-killing antibody
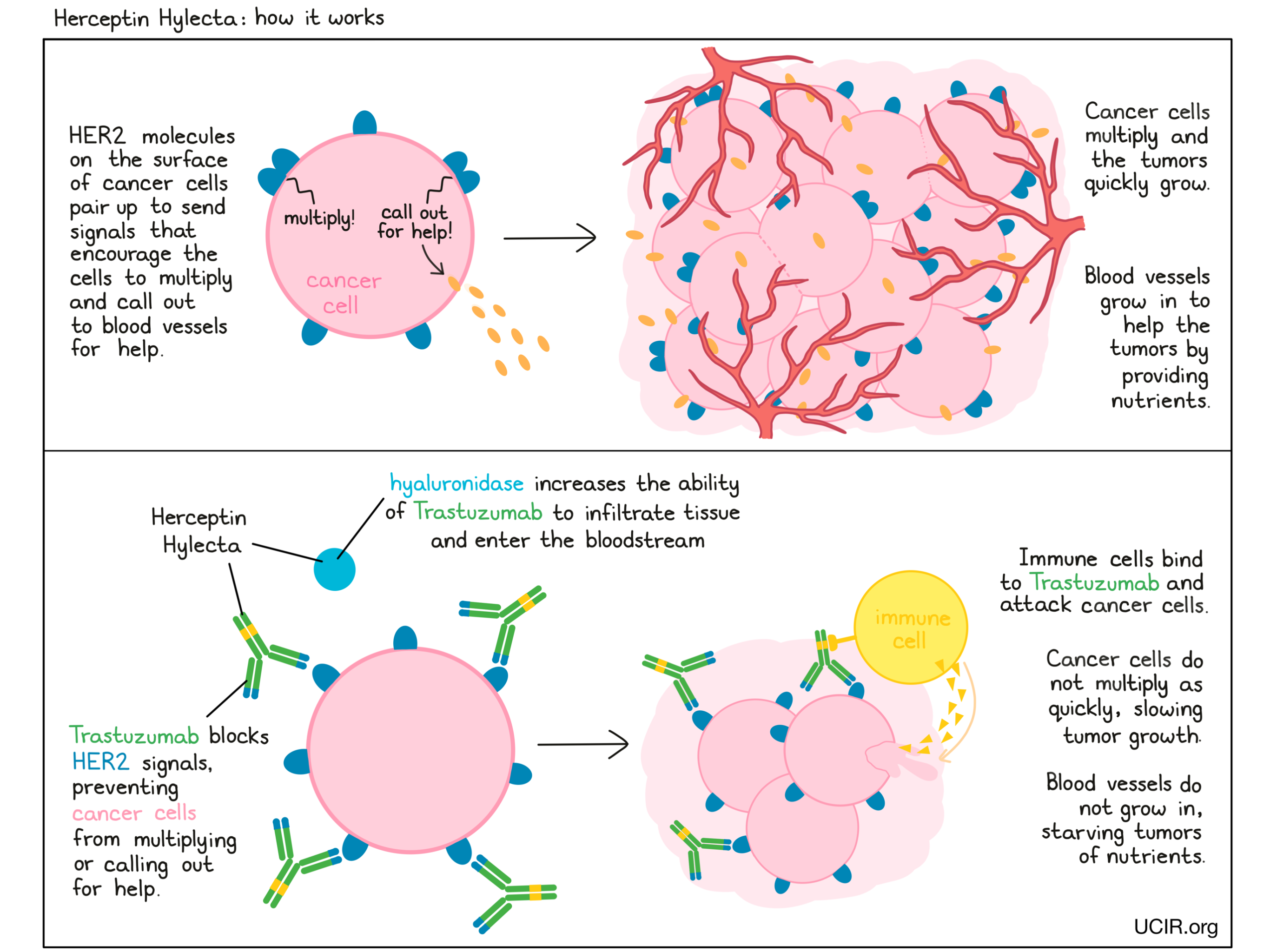
How does this drug work?
Herceptin Hylecta is a combination treatment of trastuzumab (e.g., Herceptin) and hyaluronidase. Trastuzumab is the therapeutic component, while hyaluronidase is added to increase the ability of trastuzumab to infiltrate into tissue under the skin and enter the bloodstream.
Trastuzumab is an antibody that was made in the laboratory and designed to attach to a protein molecule called HER2. HER2 is present on the surface of some normal cells in the body, but is present in much higher quantities on the surface of breast cancer cells. Higher-than-normal amounts of HER2 on cancer cells make these cells the main target of trastuzumab.
Trastuzumab and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their target. For trastuzumab, the tips of the upper arms bind to HER2. The stem of trastuzumab’s “Y” shape can attract immune cells or other parts of the immune system.
Trastuzumab works to kill cancer cells in at least two ways.
Cancer cell growth inhibition
When HER2 on the surface of cells binds to itself or to other HER2-related proteins, it sends signals into the cell. These signals cause the cell to multiply, or in other ways promote the cell’s survival, such as causing cancer cells to produce molecules that enhance the growth of blood vessels, which help feed the tumor and support its growth. Higher-than-normal amounts of HER2 allow cancer cells to grow and multiply out of control. Binding of trastuzumab to HER2 blocks HER2 from pairing up and sending these signals into the cancer cells, preventing the cells from persisting and multiplying.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to HER2 on the surface of cancer cells, the stem of trastuzumab can also attract and bind immune cells (like NK cells). This allows trastuzumab to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell trastuzumab is bound to.
How is this drug given to the patient?
Before starting treatment with Herceptin Hylecta, a small tumor sample is collected from a patient and tested to determine if the tumor is positive for high amounts of the HER2 molecule.
Herceptin Hylecta is administered via an injection under the skin (subcutaneous injection) once every 3 weeks. An injection typically takes 2-5 minutes. Patients with early breast cancer are treated for the duration of one year or until their disease comes back. For patients with advanced breast cancer, treatment with Herceptin Hylecta should continue until the disease gets worse.
Prior to the starting treatment with Herceptin Hylecta, patients undergo a thorough assessment of the condition of their heart. Patients also have their hearts monitored every 3 months during treatment with Herceptin Hylecta, and every 6 months for at least 2 years following completion of the treatment.
What are the observed clinical results?
For:
Early breast cancer
Early breast cancer (with intravenous trastuzumab)
Advanced breast cancer (previously untreated) (with intravenous trastuzumab)
Advanced breast cancer (previously treated) (with intravenous trastuzumab)
Early breast cancer
In a clinical trial, 596 patients with breast cancer that had spread within the breast or to the lymph nodes under the arms, but not to other parts of the body, or was inflammatory, and tested positive for high amounts of the HER2 molecule, were either treated with Herceptin Hylecta or intravenous trastuzumab (e.g., Herceptin) and chemotherapy before surgical removal of all known disease. Patients then continued treatment with Herceptin Hylecta or intravenous trastuzumab to complete 1 year of therapy. This trial was meant to compare the efficacy of Herceptin Hylecta with intravenous trastuzumab.

At a median follow-up of 70 months, no difference in the number of patients who were alive and had not experienced worsening of their disease was observed.
In another clinical trial, 240 patients with breast cancer that tested positive for high amounts of the HER2 molecule were treated with 4 doses of Herceptin Hylecta and 4 doses of intravenous trastuzumab (e.g., Herceptin) at a 3-week interval before or after surgical removal of all known disease. After having received all 8 doses, 231 patients completed a questionnaire:
- 86% of the patients reported preferring injection of Herceptin Hylecta under the skin over infusion of trastuzumab into a vein. The most common reason was that administration required less time in the clinic.
- 13% of the patients reported preferring infusion of trastuzumab over injection of Herceptin Hylecta. The most common reason was that they had fewer reactions at the infusion site.
- 1% of the patients had no preference for the route of administration.
Early breast cancer {#early_BC_intravenous}
Experience with intravenous trastuzumab (e.g., Herceptin)
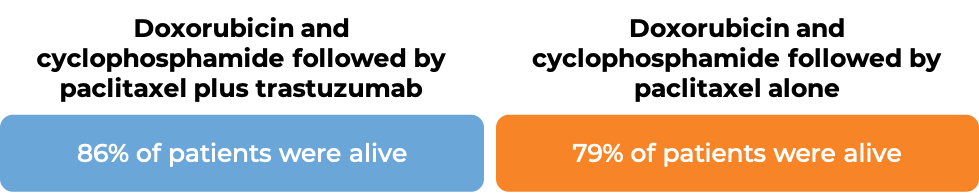
In two large clinical trials, 4063 patients with breast cancer were either treated with doxorubicin and cyclophosphamide chemotherapy followed by paclitaxel plus intravenous trastuzumab OR with doxorubicin and cyclophosphamide chemotherapy followed by paclitaxel alone to prevent the return of disease after surgical removal of all known disease.
In an analysis of 3752 patients, at a median follow-up of 2 years:

In an analysis of 4063 patients at a median follow-up of 8.3 years:
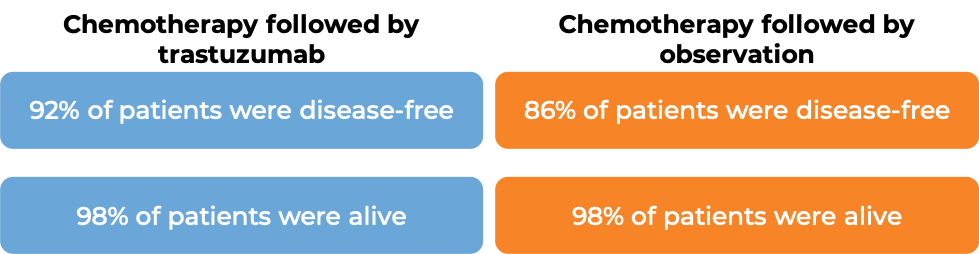
In a clinical trial, 3386 patients with breast cancer who had already been treated with chemotherapy to prevent the return of disease after surgical removal of all known disease, were either treated with intravenous trastuzumab (e.g., Herceptin) for one or two years, or were observed with no additional treatment.
At a median follow-up of 13 months:
At a median follow-up of 8 years, no added benefit in treating with trastuzumab (e.g., Herceptin) for 2 years versus 1 year was found when looking at the percentage of patients who were disease-free or the percentage of patients who were alive.
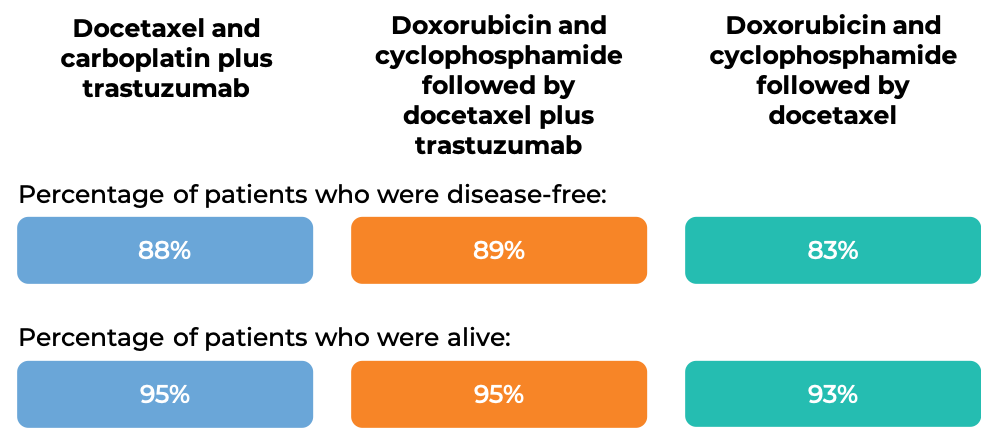
In another clinical trial, 3222 patients with breast cancer were treated with either
- Docetaxel and carboplatin plus intravenous trastuzumab, OR
- Doxorubicin and cyclophosphamide followed by docetaxel plus intravenous trastuzumab, OR
- Doxorubicin and cyclophosphamide followed by docetaxel
to prevent the return of disease after surgical removal of all known disease. At the time of data analysis:
Advanced breast cancer (previously untreated) {#early_BC_untreated}
Experience with intravenous trastuzumab (e.g., Herceptin)
In a clinical trial, 469 patients with advanced breast cancer that tested positive for high amounts of the HER2 molecule and had spread to other parts of the body, and who had not been treated for their advanced disease, were treated with chemotherapy (anthracycline [doxorubicin or epirubicin] together with cyclophosphamide OR paclitaxel) plus intravenous trastuzumab or chemotherapy alone.
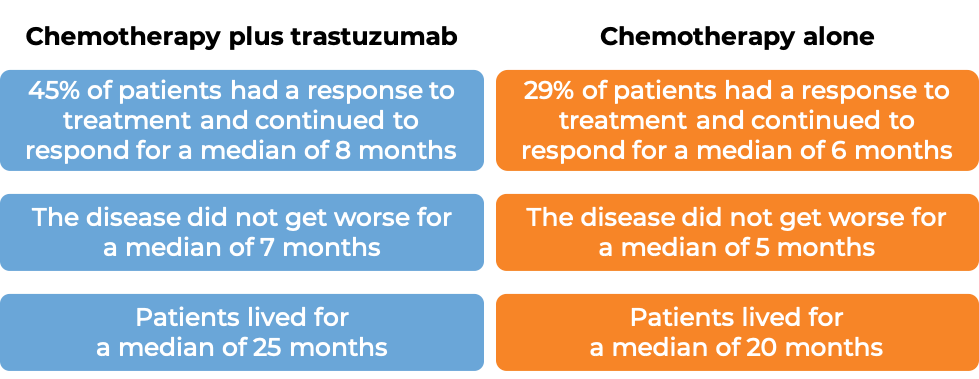
(For the definition of “median”, click HERE.)
Advanced breast cancer (previously untreated) {#advanced_BC_treated}
Experience with intravenous trastuzumab (e.g., Herceptin)
In a clinical trial, 222 patients with advanced breast cancer that tested positive for high amounts of the HER2 molecule and had spread to other parts of the body, and who had been previously treated with chemotherapy, but the disease had returned, were treated with intravenous trastuzumab alone. 12% of patients saw their tumors shrink and 2% of patients saw their tumors completely disappear. Complete disappearance of the tumors was only seen in patients with disease limited to skin and lymph nodes.
What are the potential side effects?
The most common side effects associated with Herceptin Hylecta are: fever, chills, nausea, vomiting, reactions at the injection site, diarrhea, infections, cough, headache, pain in joints, hands, and feet, fatigue, swelling of hands and feet, rash, low white and red blood cell counts, flushing, and muscle soreness.
Treatment with Herceptin Hylecta in combination with chemotherapy can cause a decrease in neutrophils, a type of immune cell that helps fight infections, making patients more likely to get an infection.
Heart problems
Herceptin Hylecta can cause potentially serious side effects, the most important of which is damage to the heart. Treatment with Herceptin Hylecta can cause a type of heart disease in which the heart is abnormally enlarged and the heart muscle becomes thickened and/or stiffened. As a result, the heart’s ability to pump blood is less efficient. The frequency of this side effect and its severity is highest in patients receiving Herceptin Hylecta together with chemotherapy containing anthracyclines, such as doxorubicin. Herceptin Hylecta can also cause other heart-related problems, including high blood pressure and changes to the heart’s rhythm. Prior to starting treatment with Herceptin Hylecta, patients undergo a thorough assessment of the condition of their heart. Patients also have their hearts monitored every 3 months during treatment with Herceptin Hylecta and upon completion of Herceptin Hylecta treatment. Treatment with Herceptin Hylecta is discontinued if severe heart issues occur.
Lung problems
Treatment with Herceptin Hylecta can cause serious lung side effects, including difficulty breathing, formation of scar tissue in the lungs, accumulation of some types of blood cells in the lungs, accumulation of fluid around the lungs, low blood oxygen levels, and more.
Hypersensitivity reactions
Hypersensitivity reactions are allergic reactions caused by the body’s immune system responding to the injection, or to components of Herceptin Hylecta as if they were a threat. Symptoms of a hypersensitivity reaction may include rashes, itching, wheezing, swelling of the face, shortness of breath, swelling of the airways, and anaphylactic shock.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer
Genentech
Approval
FDA
Links to drug websites
Last updated on May 6, 2021


