How is this drug name pronounced?
Nivolumab: nih-VOL-yoo-mab
Opdivo:op-DEE-voh
What cancer(s) does this drug treat?
Opdivo has been approved for a number of cancer types and stages, in some cases as a single therapy and in some cases in combination with ipilimumab (Yervoy), another immunotherapy.
Opdivo is approved for:
Melanoma
Lung cancer
Mesothelioma
Kidney cancer
Classical Hodgkin lymphoma
Head and neck squamous cell cancer
Bladder and urinary tract (urothelial cell) cancer
Colorectal cancer (MSI-H/dMMR)
Liver cancer
Esophageal cancer
Stomach cancer
Advanced melanoma
Opdivo is approved for:
- Adult and pediatric (12 years or older) patients with advanced melanoma that is metastatic (cancer has spread to other parts of the body from the original cancer site) or cannot be completely removed by surgery. Opdivo may be used by itself or in combination with ipilimumab (Yervoy).
- Adult and pediatric (12 years or older) patients with melanoma (Stage IIB, IIC, III, or IV) that, together with lymph nodes or other tissue the cancer has spread to (metastases), was completely removed by surgery. In such cases, Opdivo is used to help keep melanoma from coming back.
Advanced lung cancer
Opdivo is approved for:
- Adult patients with non-small cell lung cancer that can be removed by surgery. In such cases, Opdivo is used in combination with platinum-containing therapy prior to surgery, in an effort to reduce the size of the tumor.
- Adult patients with non-small cell lung cancer that has spread, and whose tumors test positive for the PD-L1 molecule and do not have an abnormal EGFR or ALK gene. In such cases, Opdivo is used in combination with ipilimumab (Yervoy) as a first treatment.
- Adult patients with non-small cell lung cancer that has spread or come back and whose tumors do not have an abnormal EGFR or ALK gene. In such cases, Opdivo is used in combination with ipilimumab (Yervoy) and two treatment cycles of platinum-containing chemotherapy as a first treatment.
- Adult patients with non-small cell lung cancer that has spread, who have tried chemotherapy containing platinum, and it either did not work or stopped working. If the patient’s tumor has an abnormal EGFR or ALK gene, the patient has to have also tried an FDA-approved therapy for tumors with such abnormal genes prior to receiving Opdivo.
Mesothelioma
Opdivo is approved for:
- Adult patients with newly diagnosed malignant pleural mesothelioma (a cancer in the cells that line the inside of the chest and cover the lungs) whose cancer could not be removed by surgery may be treated with Opdivo in combination with ipilimumab (Yervoy).
Advanced kidney cancer
Opdivo is approved for:
- Adult patients with renal cell carcinoma (kidney cancer) that has grown or spread, and who have previously been treated with an angiogenesis inhibitor.
- Adult patients with renal cell carcinoma that has grown or spread, and who have not received any other treatment for their advanced disease. In such cases, Opdivo may be used in combination with cabozantinib (e.g., Cabometyx).
- Adult patients with renal cell carcinoma that has grown or spread who have certain risk factors and who have not received any other treatment for their advanced disease. In such cases, Opdivo may be used in combination with ipilimumab (Yervoy).
Classical Hodgkin lymphoma
Opdivo is approved for:
- Adult patients with classical Hodgkin lymphoma that has come back or spread after autologous stem cell transplant (a type of transplant that uses the patient’s own stem cells) and either have received brentuximab vedotin (Adcetris)or have been treated with at least 3 kinds of cancer treatments (including the autologous stem cell transplant).
Head and neck squamous cell cancer
Opdivo is approved for:
- Adult patients with head and neck squamous cell carcinoma whose cancer has come back or spread after treatment with chemotherapy that contains platinum.
Advanced bladder and urinary tract (urothelial cell) cancer
Opdivo is approved for:
- Adult patients with advanced urothelial carcinoma (the most common type of bladder and urinary tract cancer) that has grown or spread and who have been treated with chemotherapy containing platinum, and it did not work or stopped working.
- Adult patients with urothelial carcinoma who are at high risk of their cancer coming back after surgical removal of all known disease.
- Adult patients with urothelial carcinoma that cannot be removed by surgery or has spread to other parts of the body, and who have not yet received treatment for their advanced disease. In such cases, Opdivo is used in combination with cisplatin and gemcitabine chemotherapy.
Advanced colorectal cancer (MSI-H/dMMR)
Opdivo is approved for:
- Adult and pediatric patients 12 years of age and older with colon or rectal cancer that has spread, have a tumor that is microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR), and have been treated with a fluoropyrimidine, oxaliplatin, and irinotecan chemotherapy, and the treatment did not work or stopped working. Patients may be treated with Opdivo alone or in combination with ipilimumab (Yervoy).
Advanced liver cancer
Opdivo is approved for:
- Adult patients with hepatocellular carcinoma (liver cancer) who have been treated with sorafenib (Nexavar). In such cases, Opdivo is used in combination with ipilimumab (Yervoy).
Advanced esophageal cancer
Opdivo is approved for:
- Adult patients with esophageal cancer or gastroesophageal junction cancer that has been completely removed by surgery after being treated by chemotherapy and concurrent radiation, but cancer cells remain in the body after treatment.
- Adult patients with esophageal squamous cell carcinoma that is advanced and cannot be removed by surgery or has spread to other parts of the body, and who have not yet received treatment for their advanced disease. In such cases, Opdivo is used in combination with a fluoropyrimidine- and platinum-containing chemotherapy OR in combination with ipilimumab (Yervoy).
- Patients with esophageal squamous cell carcinoma that is advanced and cannot be removed by surgery, has come back, or has spread to other parts of the body, and who have been previously treated with fluoropyrimidine- and platinum-containing chemotherapy. In such cases, Opdivo is used by itself.
- Adult patients with esophageal adenocarcinoma, gastroesophageal junction cancer or gastric (stomach) cancer that has grown or spread to other parts of the body. In such cases, Opdivo is used in combination with fluoropyrimidine and platinum-containing chemotherapy.
Advanced stomach cancer
Opdivo is approved for:
- Adult patients with stomach (gastric) cancer, gastroesophageal junction or esophageal adenocarcinoma that has grown or spread to other parts of the body. In such cases, Opdivo is used in combination with fluoropyrimidine- and platinum-containing chemotherapy.
Limitations of use
Age: The safety and efficacy of Opdivo in patients with MSI-H/dMMR metastatic colorectal cancer or melanoma under 12 years of age have not been established. The safety and efficacy of Opdivo in patients under 18 years of age with all other cancer types approved for treatment with Opdivo have not been established.
Pregnancy/Breastfeeding: Opdivo can cause harm to a fetus, and is not recommended for use during pregnancy. Women are advised to use contraception during treatment, and for at least 5 months after the last dose of Opdivo. The risks associated with Opdivo during breastfeeding are not known and cannot be ruled out; due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for 5 months after the last dose of Opdivo.
Complications of stem cell transplant: Serious and life-threatening complications that can lead to death can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Opdivo. The benefit of treatment with Opdivo versus the possible risk of transplant-related complications (especially in patients with a history of graft-versus-host disease) should be carefully considered.
What type of immunotherapy is this?
- PD-1 blockade
How does this drug work?
- Target: PD-1
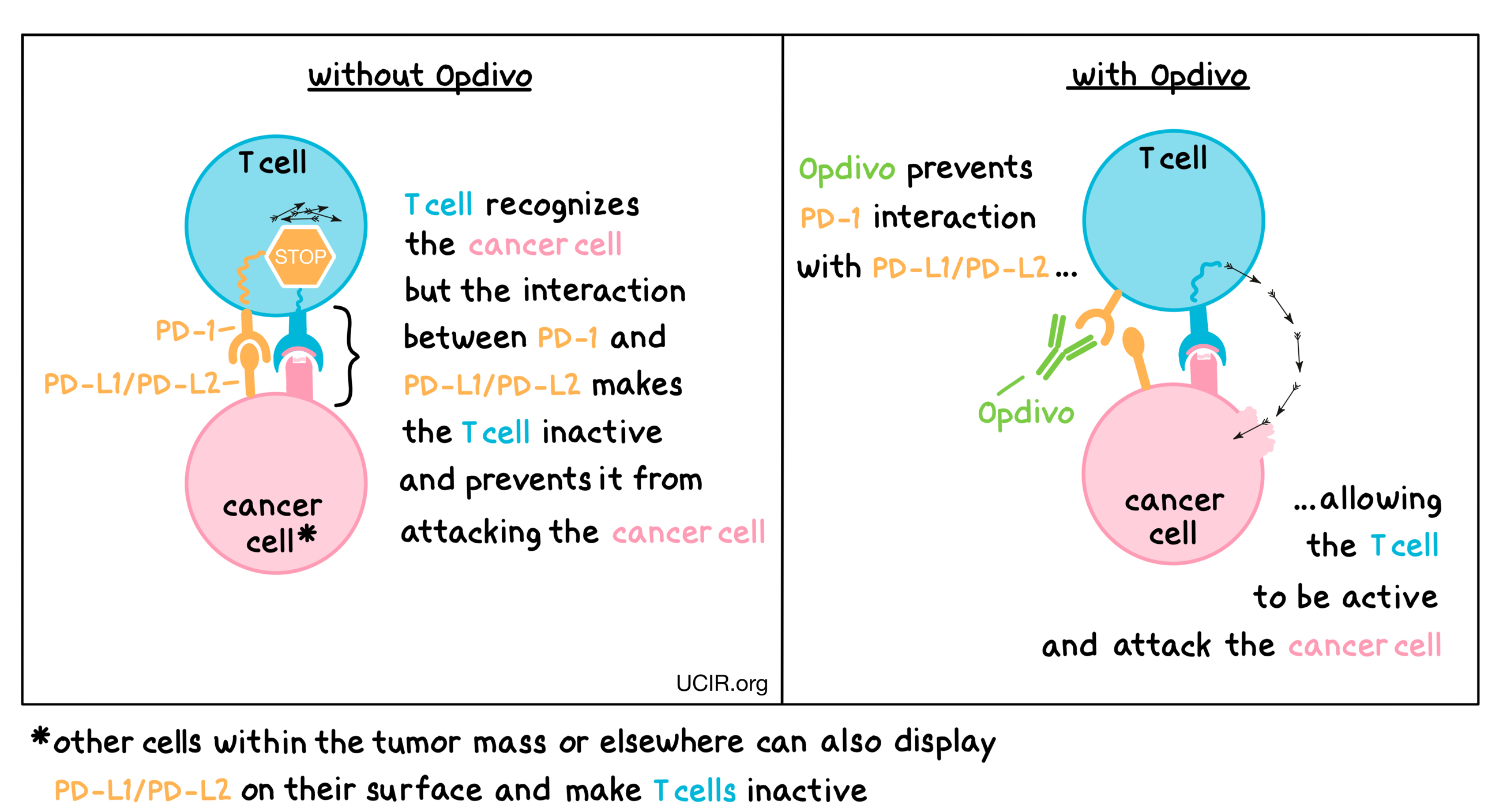
Opdivo is an antibody that attaches to a molecule called PD-1, which is present on the surface of T cells – the primary immune cells involved in killing cancer cells. In healthy tissues, PD-1 acts as a brake that keeps T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from doing their job – killing the cancer cells. This can happen when cancer cells or other cells within the tumor mass display on their surface molecules called PD-L1 or PD-L2. When PD-L1 or PD-L2 interact with PD-1 on T cells, the T cells become inactive and do not attack the cancer cells. Opdivo binds to the PD-1 molecules on T cells in such a way that it prevents the interaction between PD-1 and PD-L1/PD-L2, and allows the T cells to be active and attack the cancer cells.
How is this drug given to the patient?
Opdivo is administered via a tube into a vein (intravenous infusion, or I.V.) over 30 minutes and does not require a hospital stay. Opdivo is usually given to the patient either every two, three, or four weeks, depending on the dose, the combination with other drugs, and the cancer type.
What are the observed clinical results?
For:
Advanced melanoma (metastatic or not removable by surgery)
Advanced melanoma (completely removed by surgery)
Advanced non-small cell lung cancer (squamous and non-squamous)
Advanced kidney cancer (previously treated or untreated)
Classical Hodgkin lymphoma
Head and neck squamous cell cancer
Advanced bladder and urinary tract cancer
Advanced colorectal cancer (MSI-H/dMMR)
Advanced liver cancer
Advanced esophageal cancer
Advanced stomach cancer
Malignant pleural mesothelioma
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies described below. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced melanoma (metastatic or not removable by surgery)
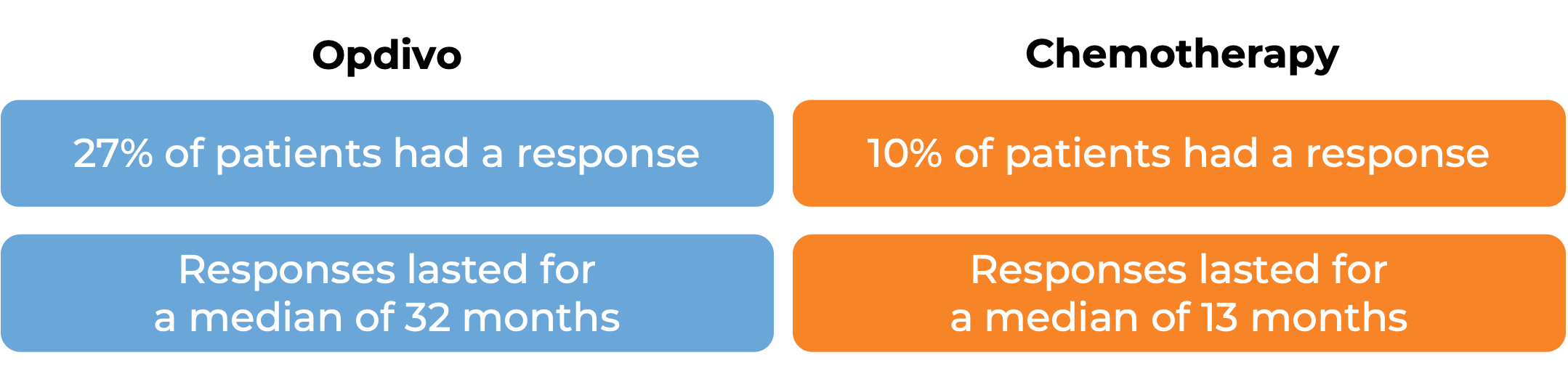
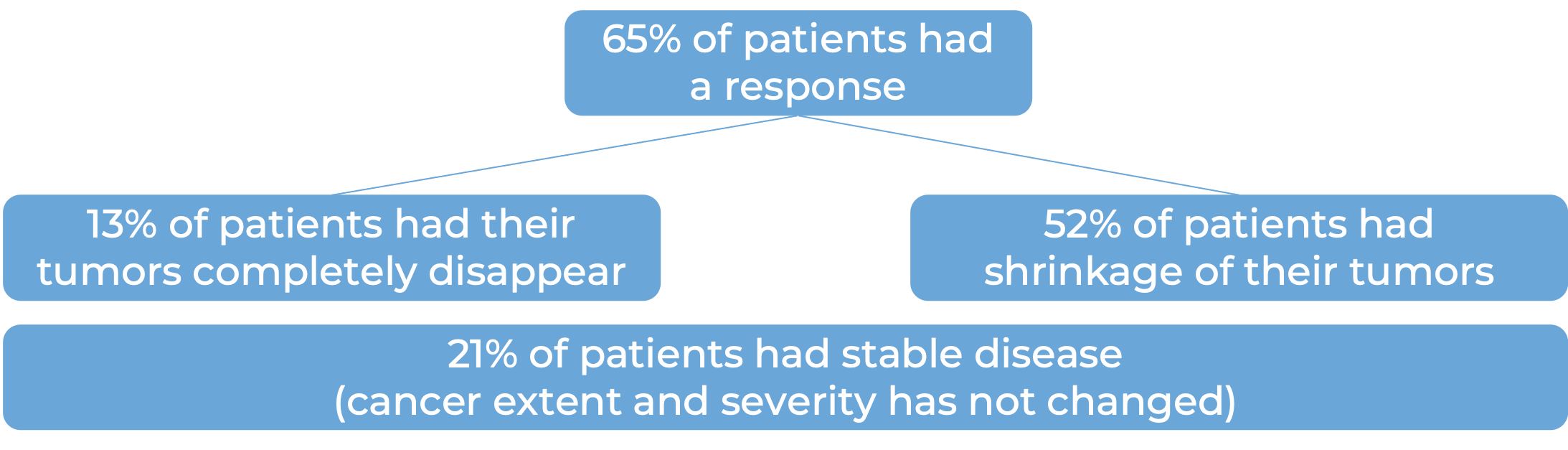
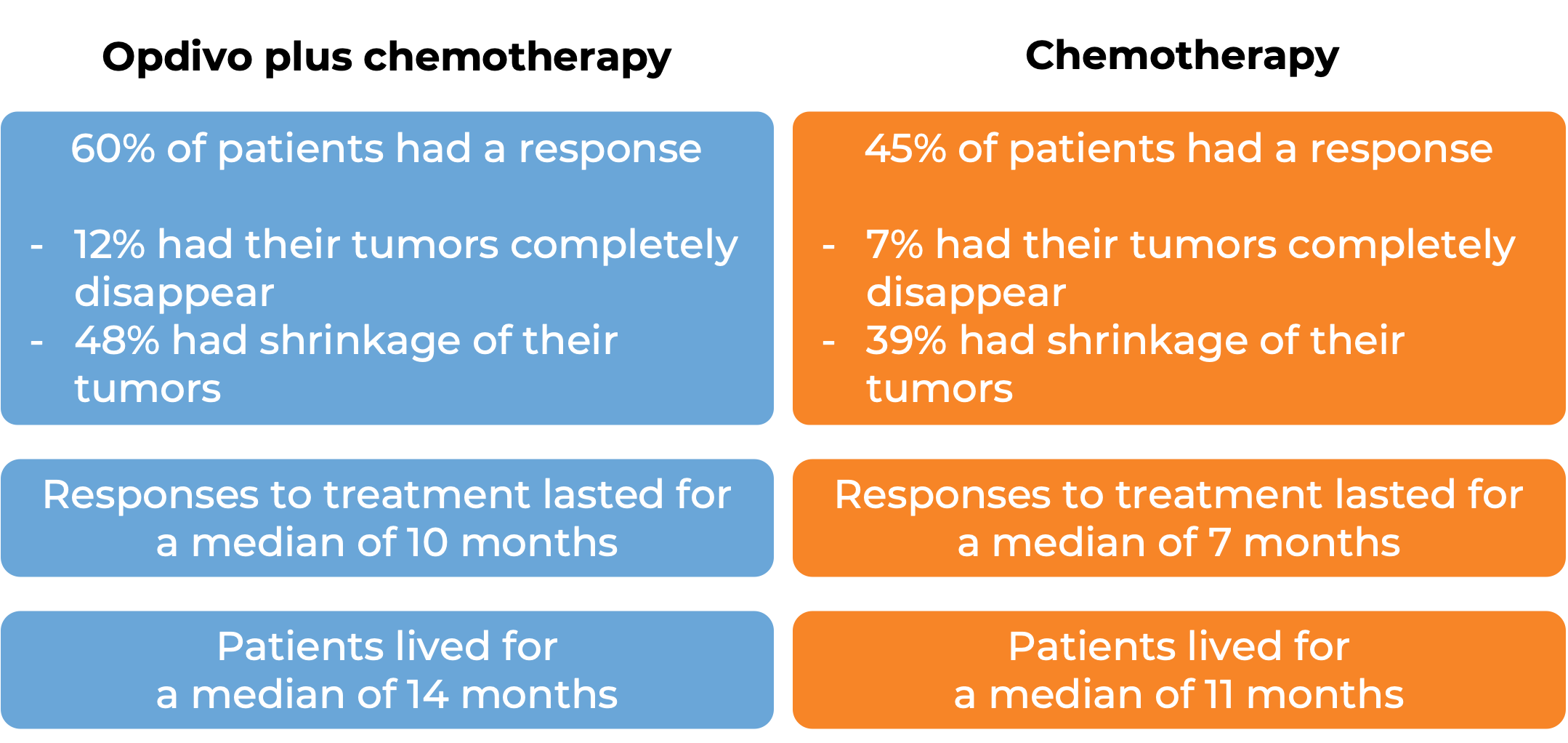
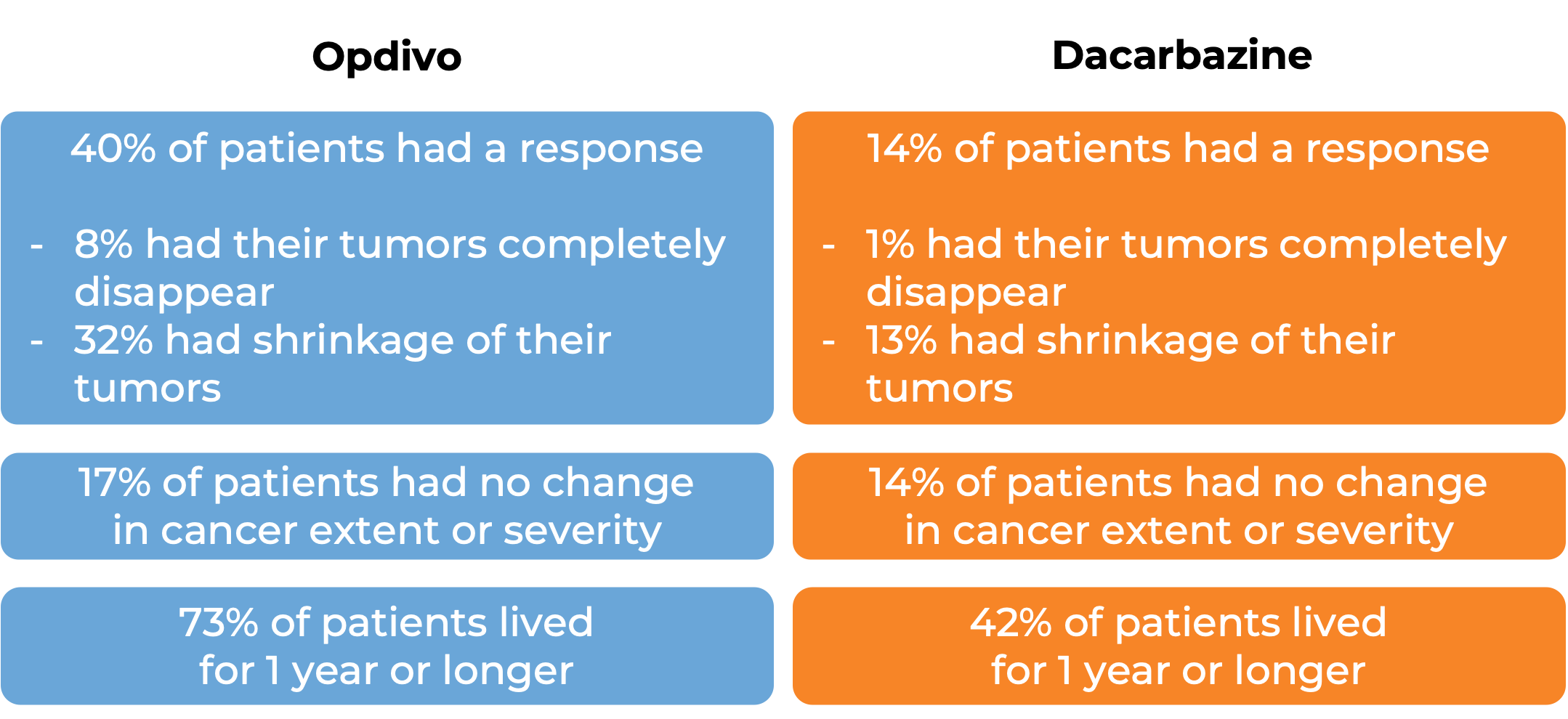
In a clinical trial, 418 patients with melanoma that had spread to other parts of the body or could not be removed by surgery were treated with Opdivo or dacarbazine. At a median follow-up of 17 months:
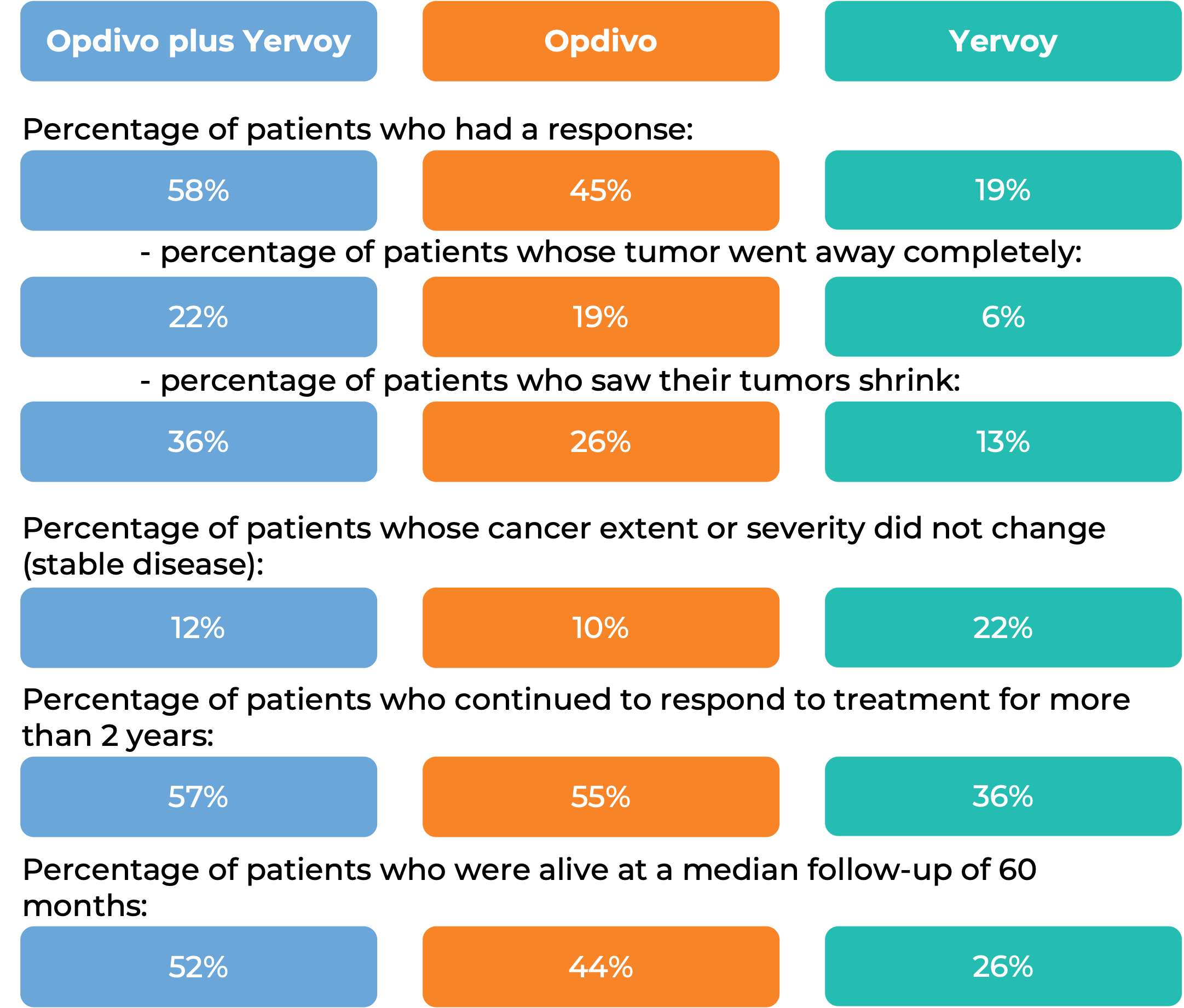
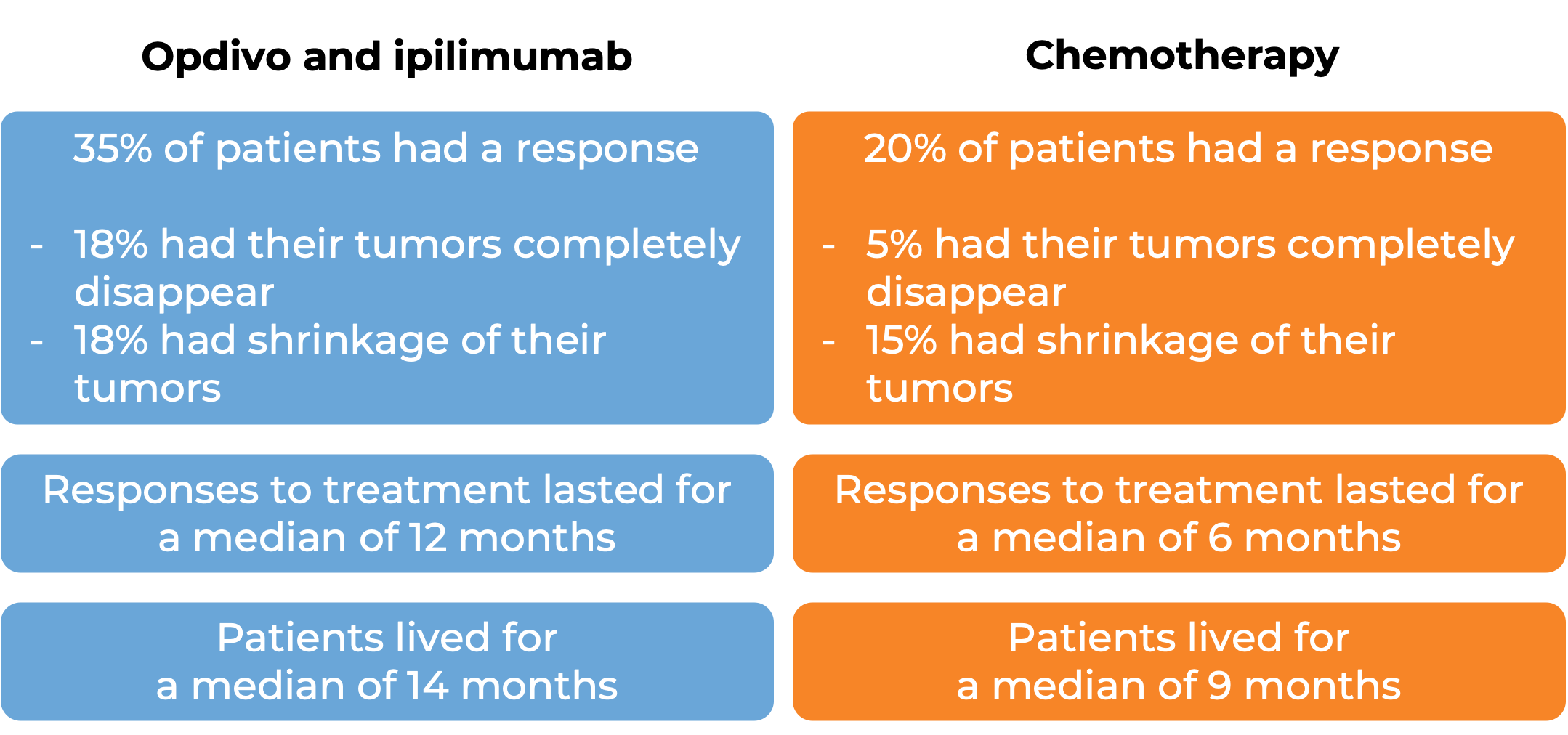
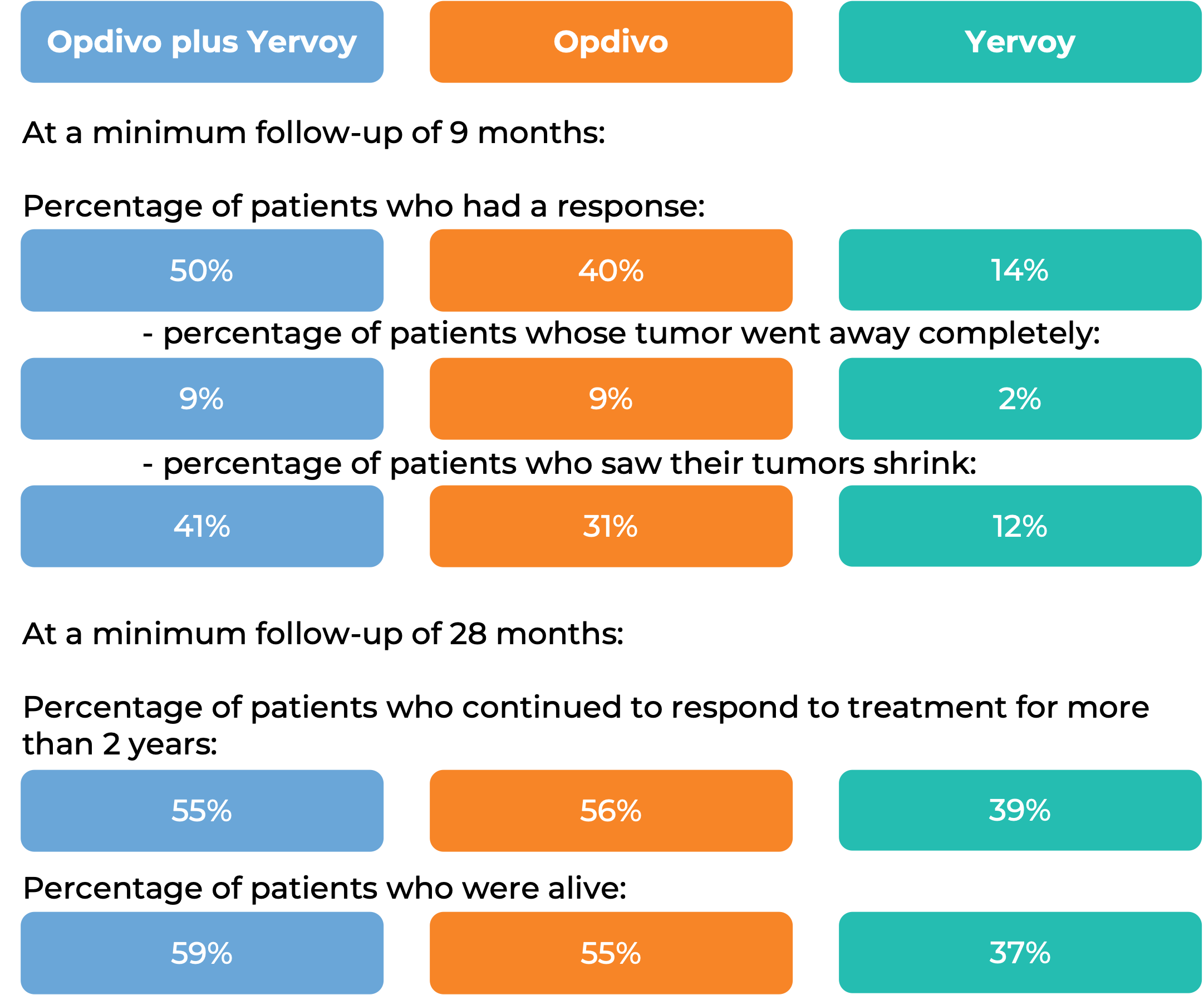
In another clinical trial, 945 patients with melanoma that had spread to other parts of the body or could not be removed by surgery, and who had not received treatment for their advanced disease were treated with Opdivo plus ipilimumab (Yervoy), Opdivo alone, or Yervoy alone.
In another clinical trial, 405 patients with melanoma that had spread to other parts of the body or could not be removed by surgery and whose disease had gotten worse or come back during or after treatment with ipilimumab (Yervoy), were treated with Opdivo or chemotherapy (dacarbazine or carboplatin plus paclitaxel).

(For the definition of “median” click HERE.)
Advanced melanoma (completely removed by surgery)
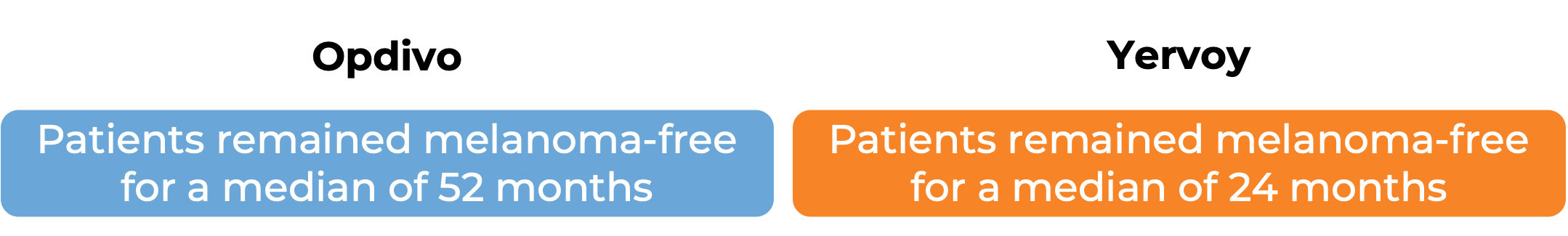
In a clinical study, 906 patients with Stage IIIB/C or IV melanoma that was completely removed by surgery were treated with either Opdivo or ipilimumab (Yervoy) to prevent the cancer from coming back. At a minimum follow-up of 62 months:
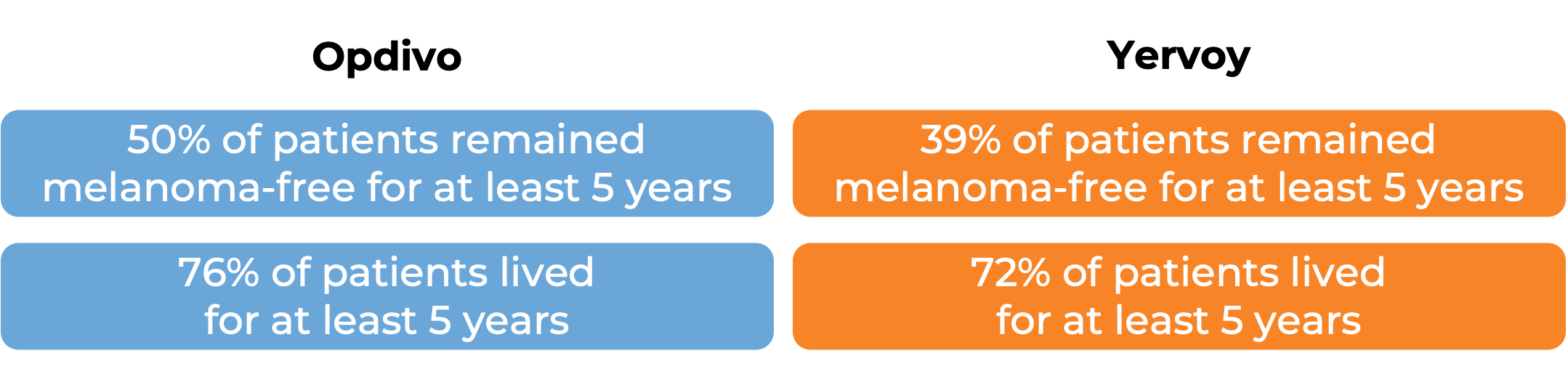
(For the definition of “median” click HERE.)
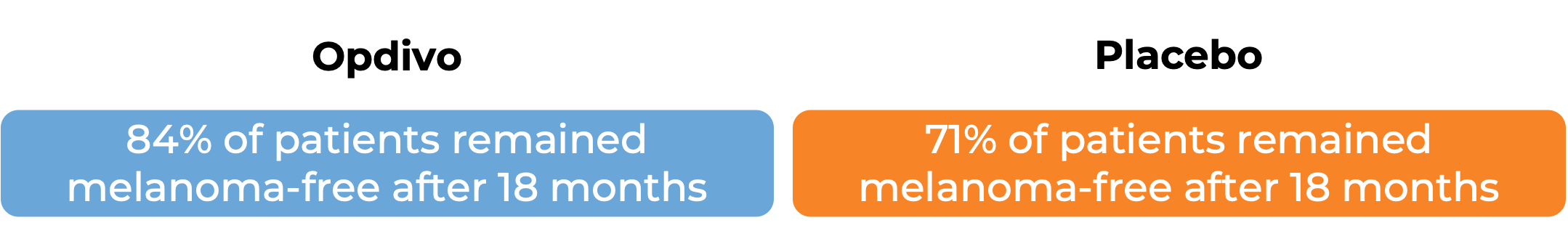
In another clinical trial, 790 patients with Stage IIB/C melanoma that was completely removed by surgery were treated with either Opdivo or a placebo to prevent the cancer from coming back.

Advanced non-small cell lung cancer (squamous and non-squamous)
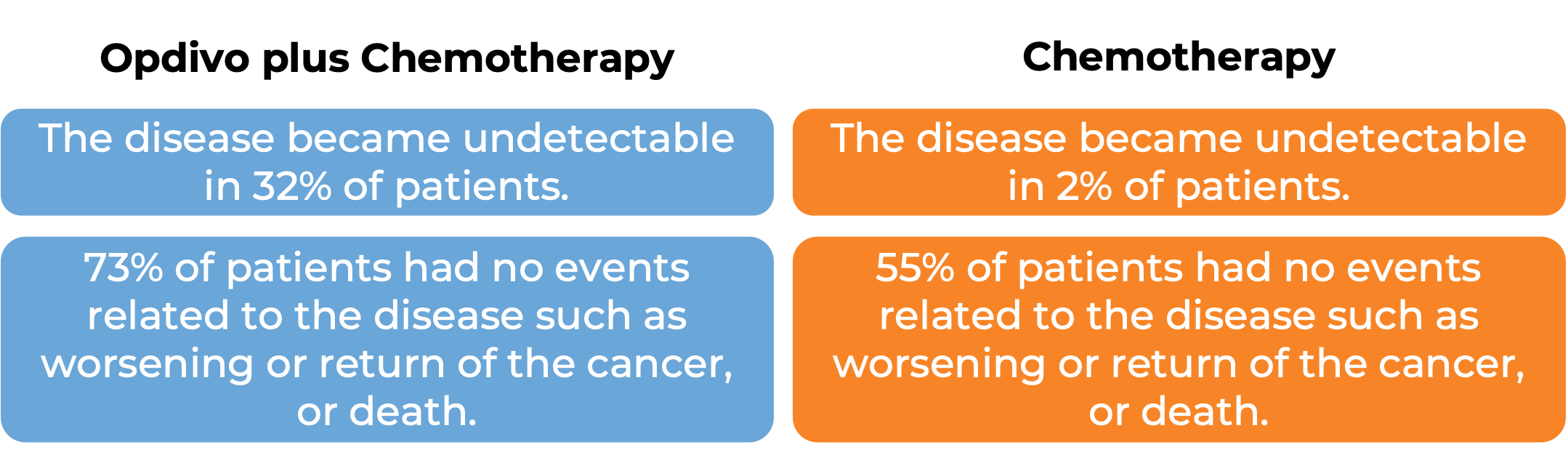
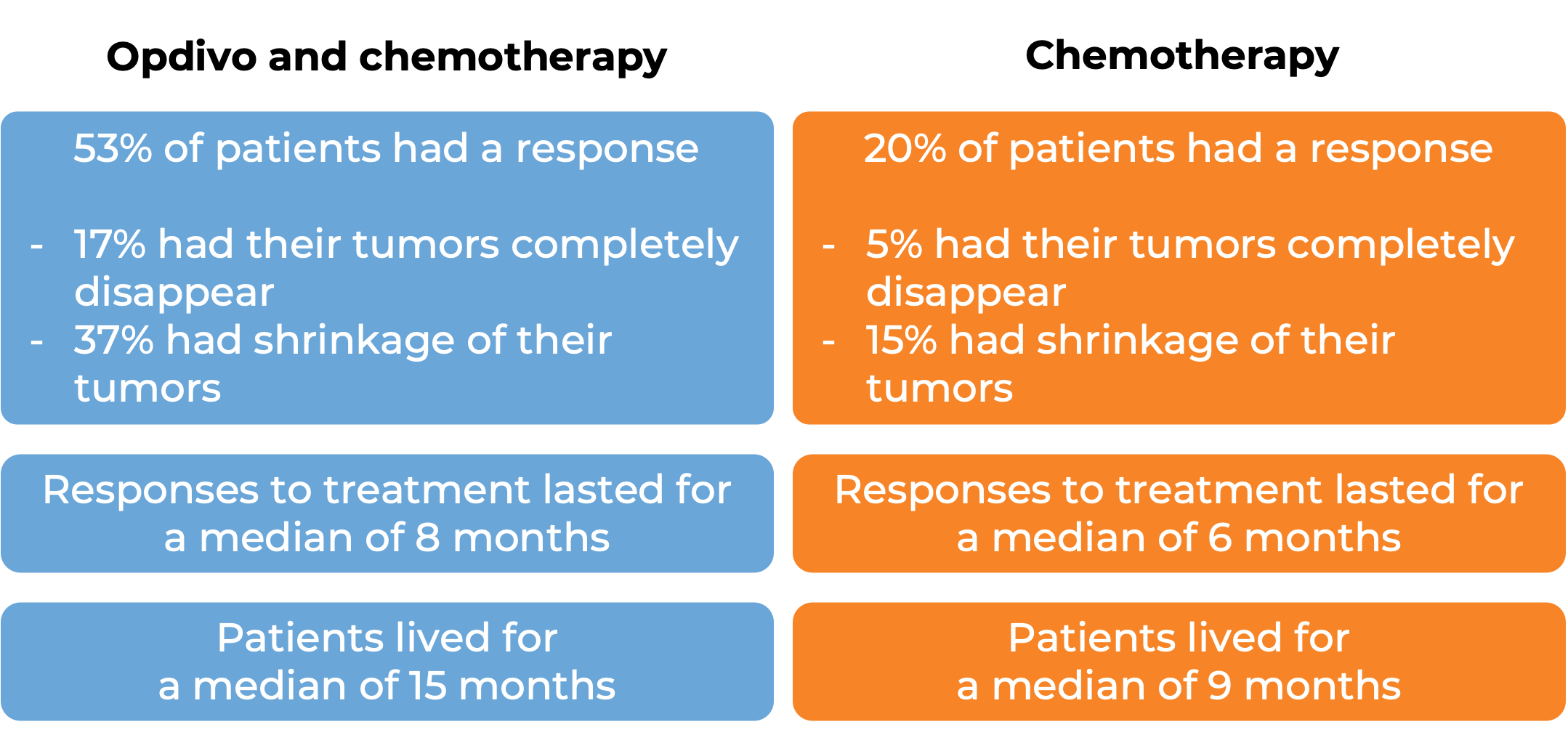
In a clinical study, 358 patients with non-small cell lung cancer that could be removed by surgery were treated with either Opdivo in combination with platinum-containing chemotherapy, or with platinum-containing chemotherapy alone prior to surgery to remove their cancer. At a minimum follow-up of 21 months:
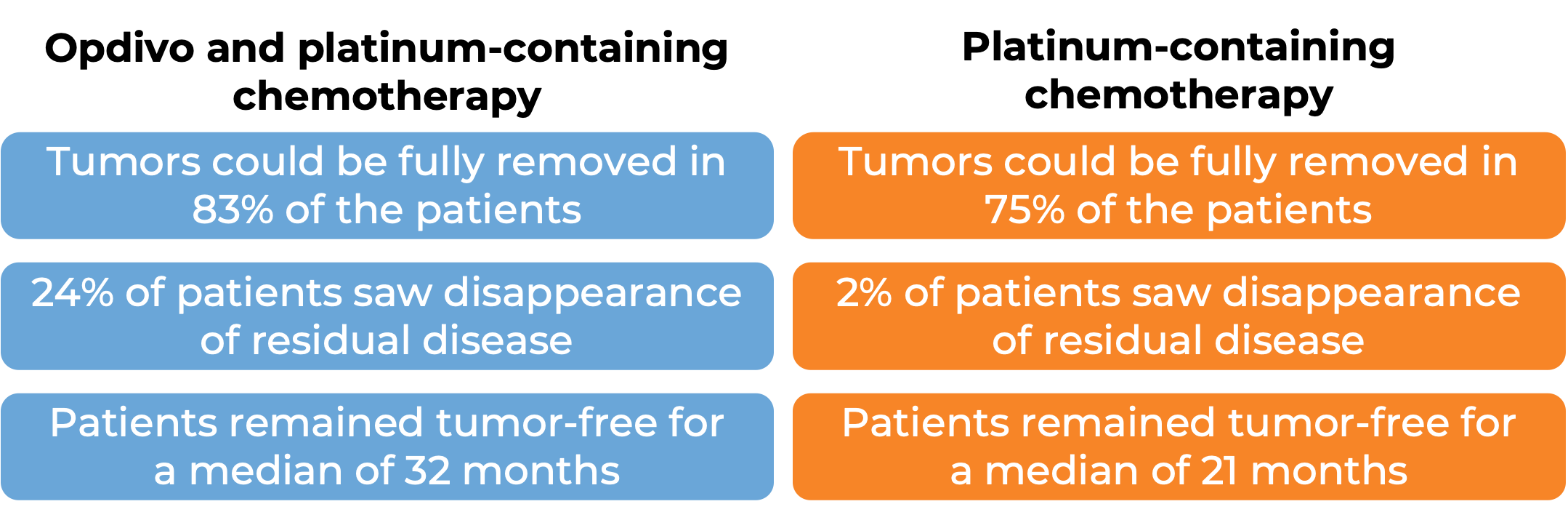
(For the definition of “median” click HERE.)
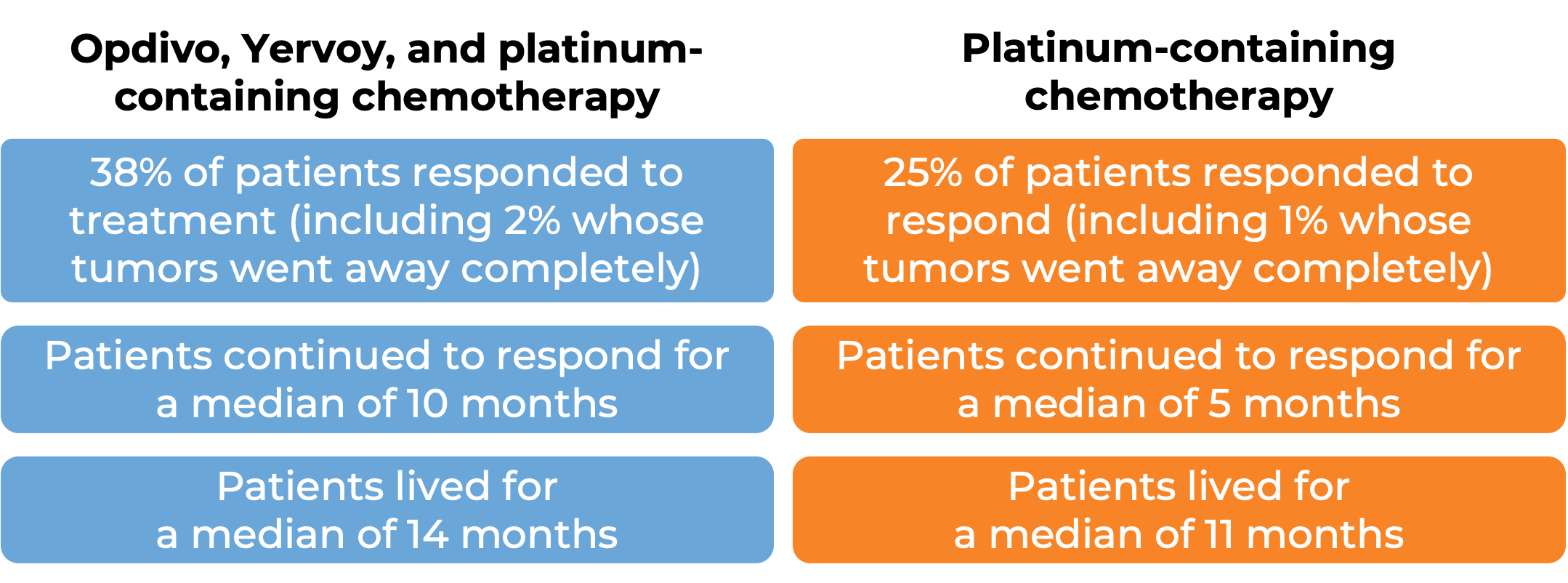
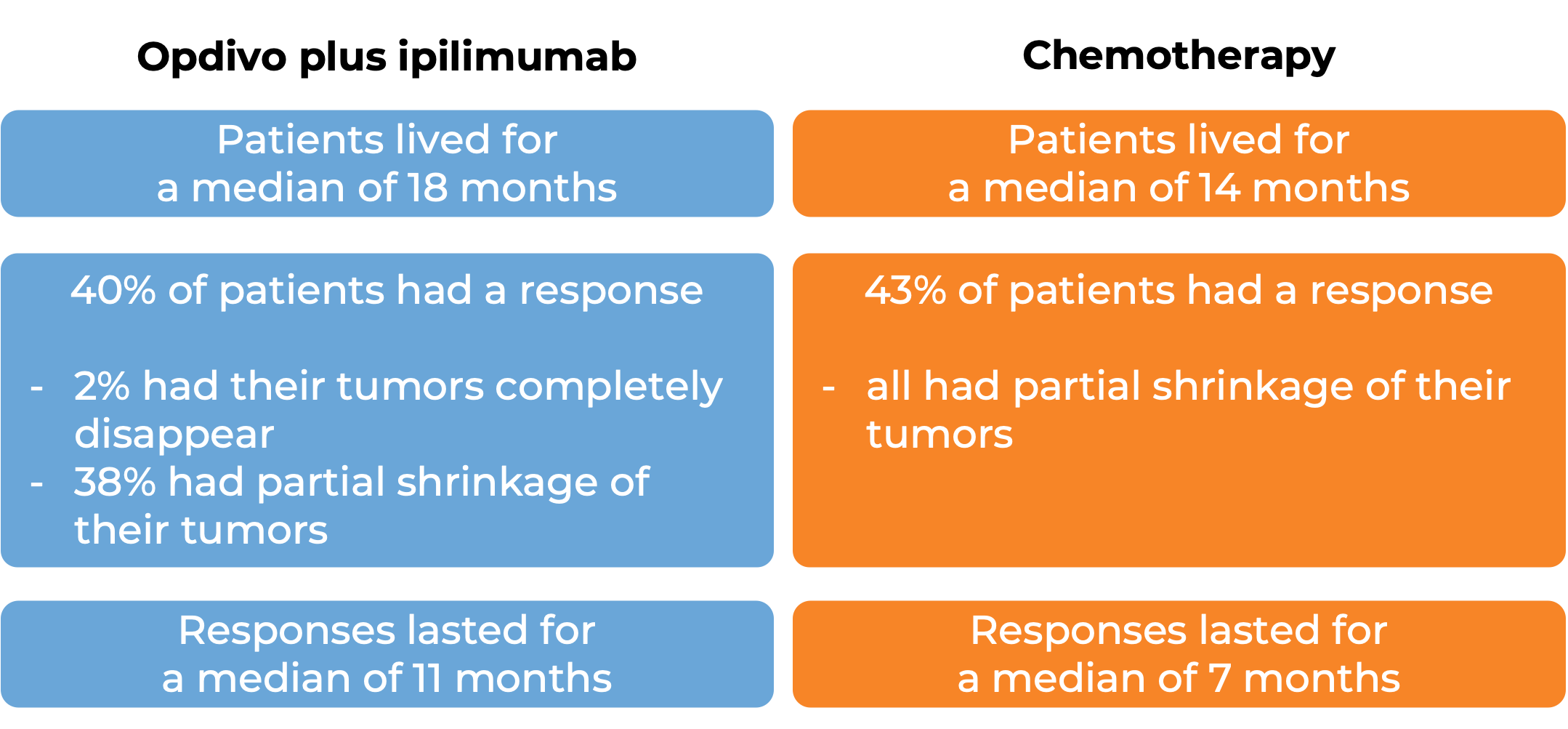
In a clinical study of patients with non-small cell lung cancer that had spread or come back, who had not yet received treatment for their advanced disease and whose tumors did not have an abnormal EGFR or ALK gene, were treated with either Opdivo plus ipilimumab (Yervoy) OR platinum-containing chemotherapy. At a minimum follow-up of 29 months, among the 793 patients whose tumors tested positive for the PD-L1 molecule:
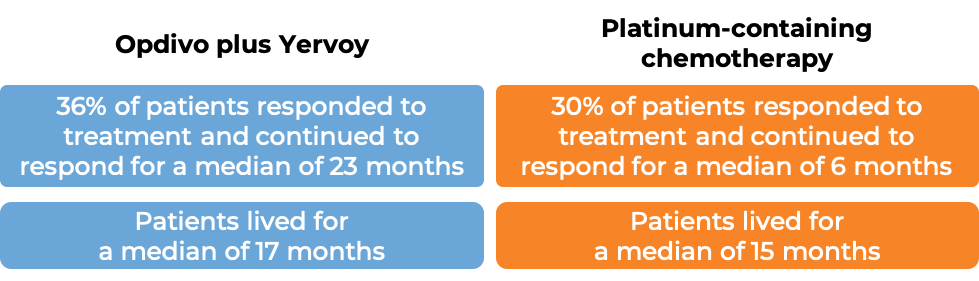
(For the definition of “median” click HERE.)
In another clinical study of 719 patients with previously untreated non-small cell lung cancer that had spread or come back, whose tumors did not have an abnormal EGFR or ALK gene, and who had not received treatment for their advanced disease, were treated with either:
- Opdivo, ipilimumab, and platinum-containing chemotherapy, OR
- platinum-containing chemotherapy.
At a minimum follow-up of 13 months:
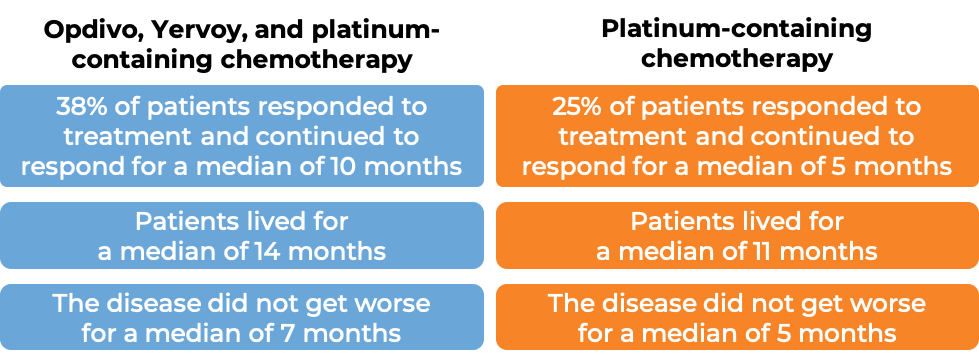
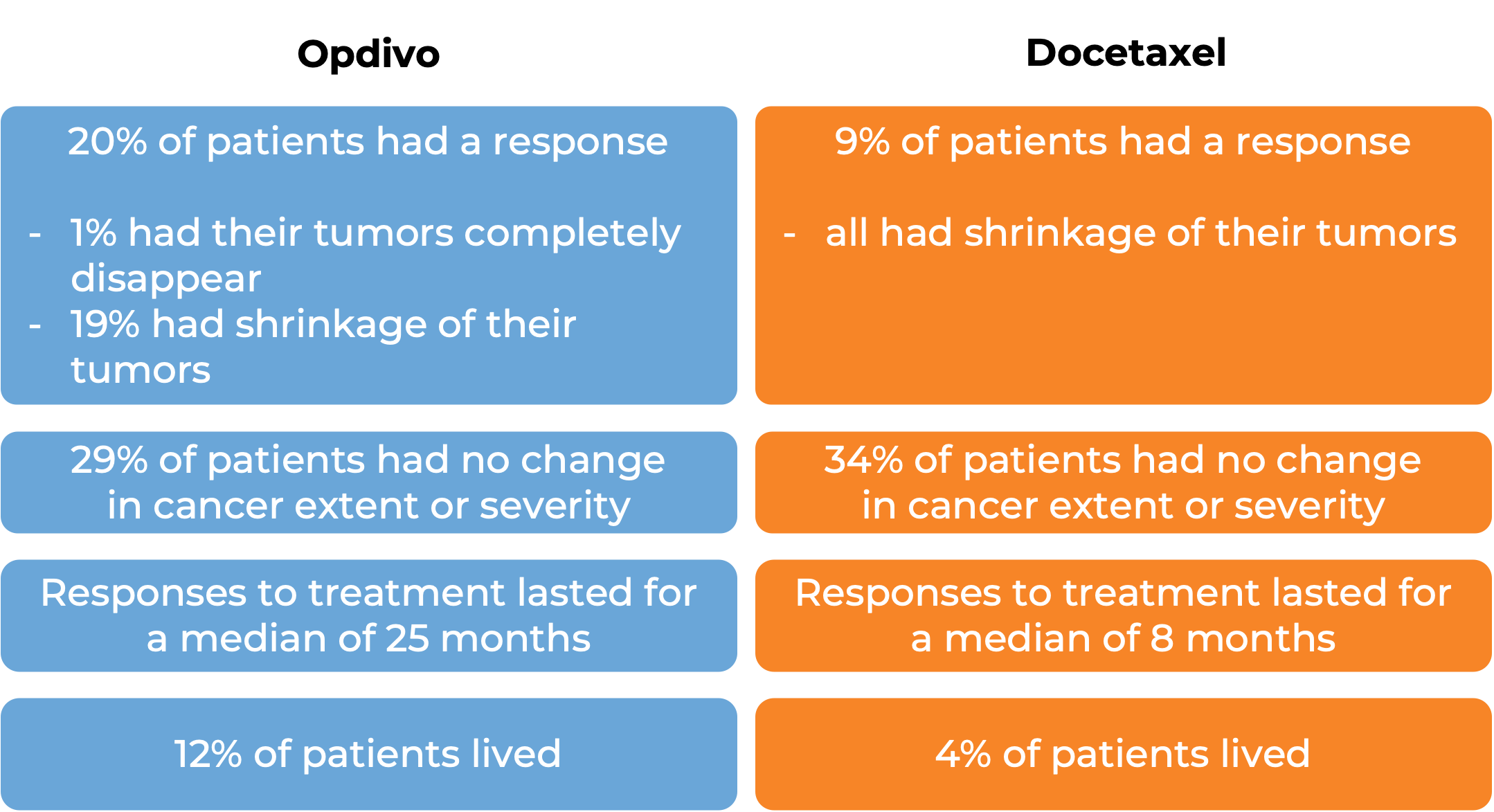
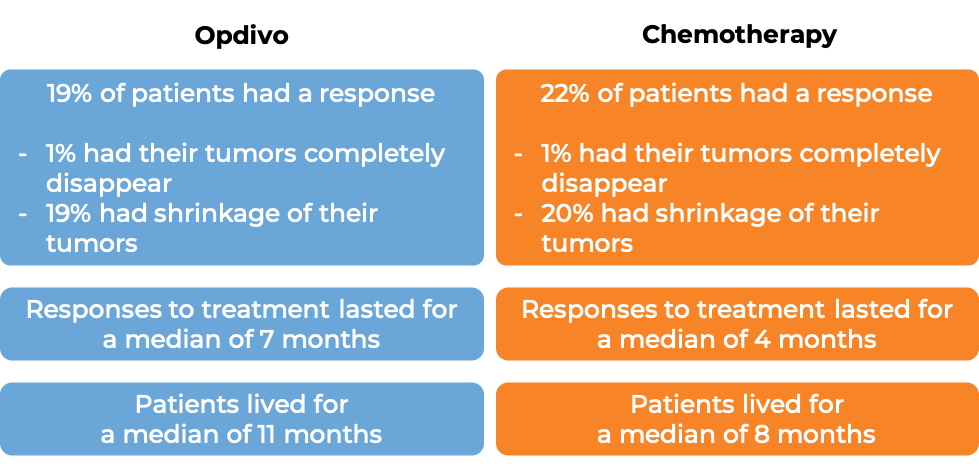
In a clinical study, 272 patients with previously treated squamous non-small cell lung cancer that had spread to other parts of the body were treated with either Opdivo or docetaxel (a chemotherapy):
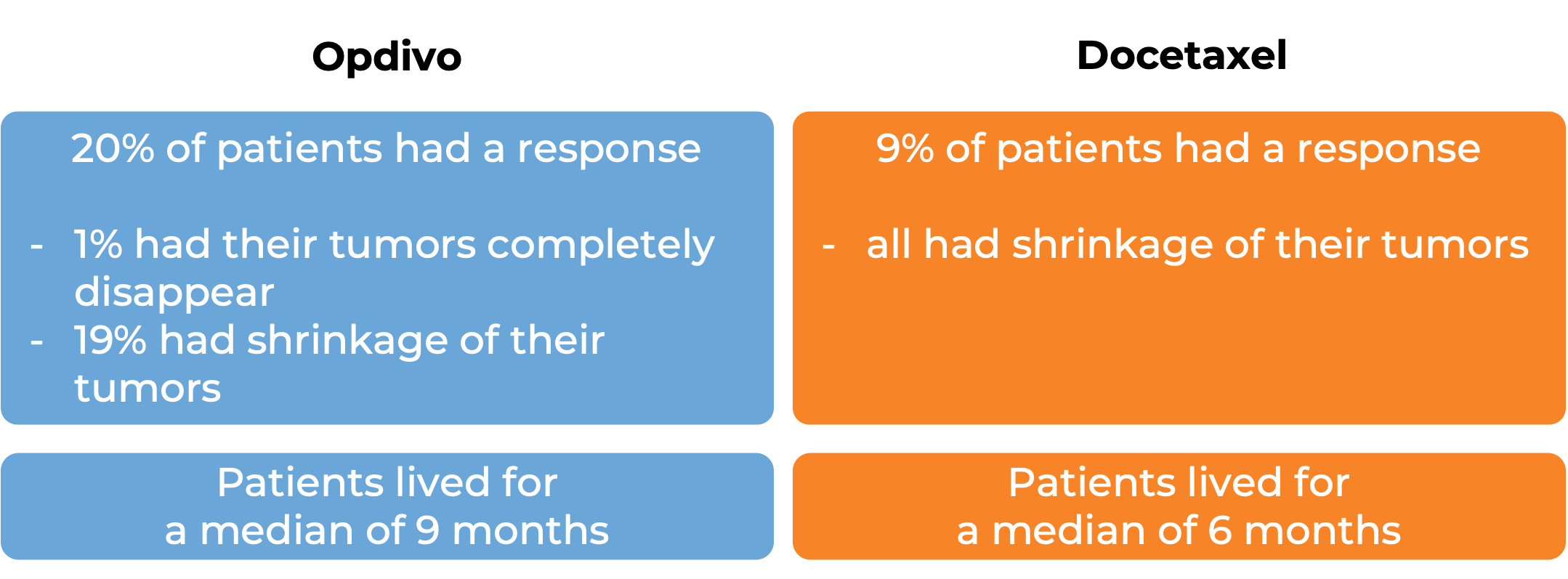
In another clinical study, 582 patients with previously treated non-squamous non-small cell lung cancer that had grown or spread to other parts of the body were treated with either Opdivo or docetaxel (a chemotherapy):
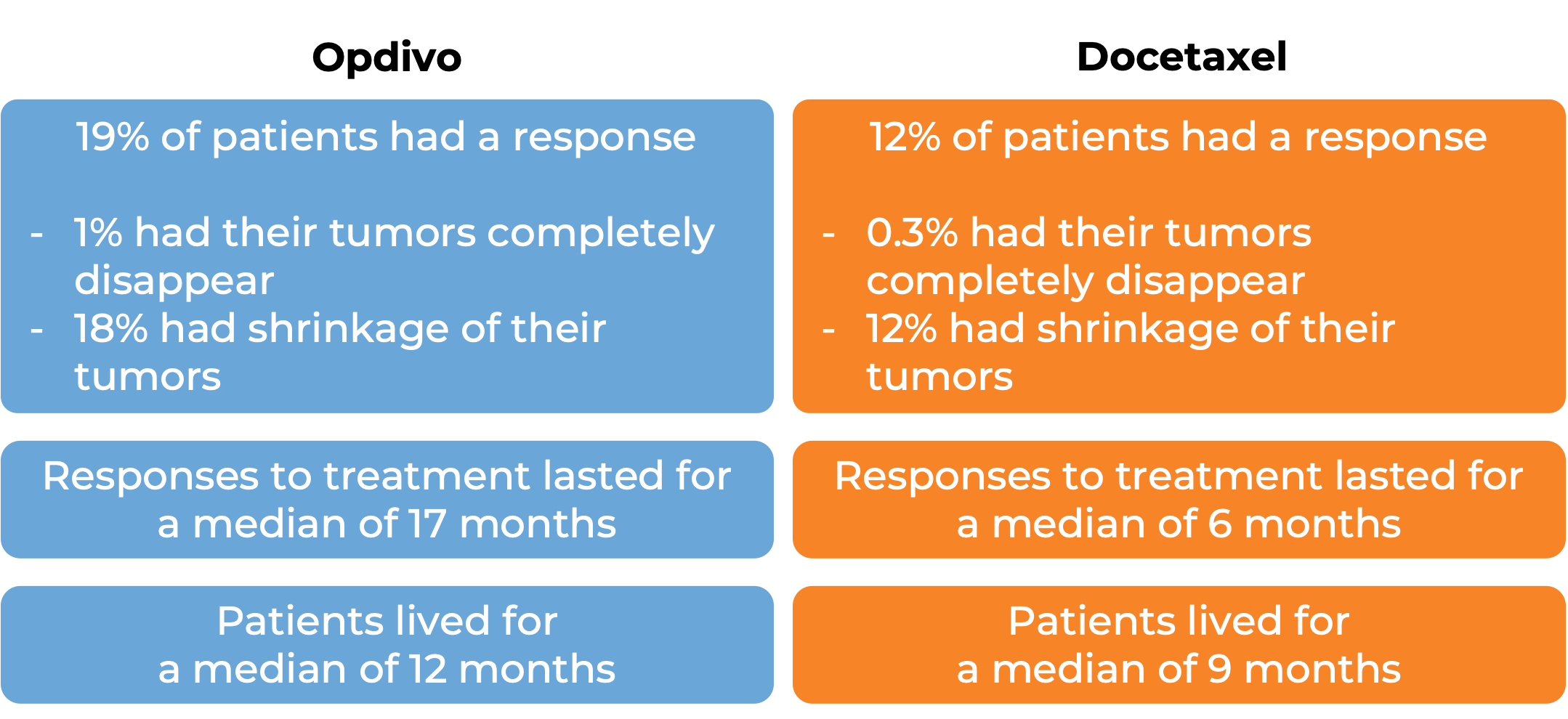
Advanced kidney cancer (previously treated or untreated)
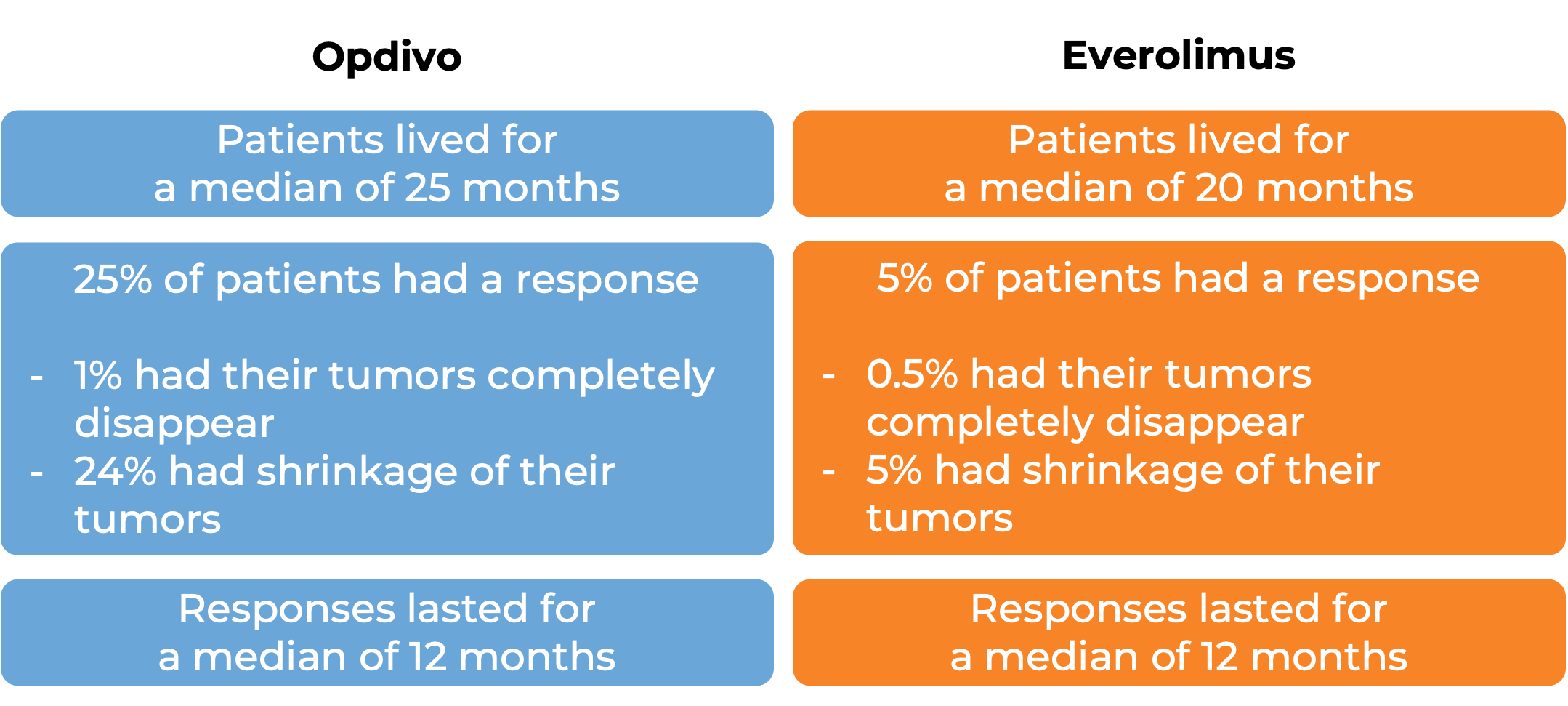
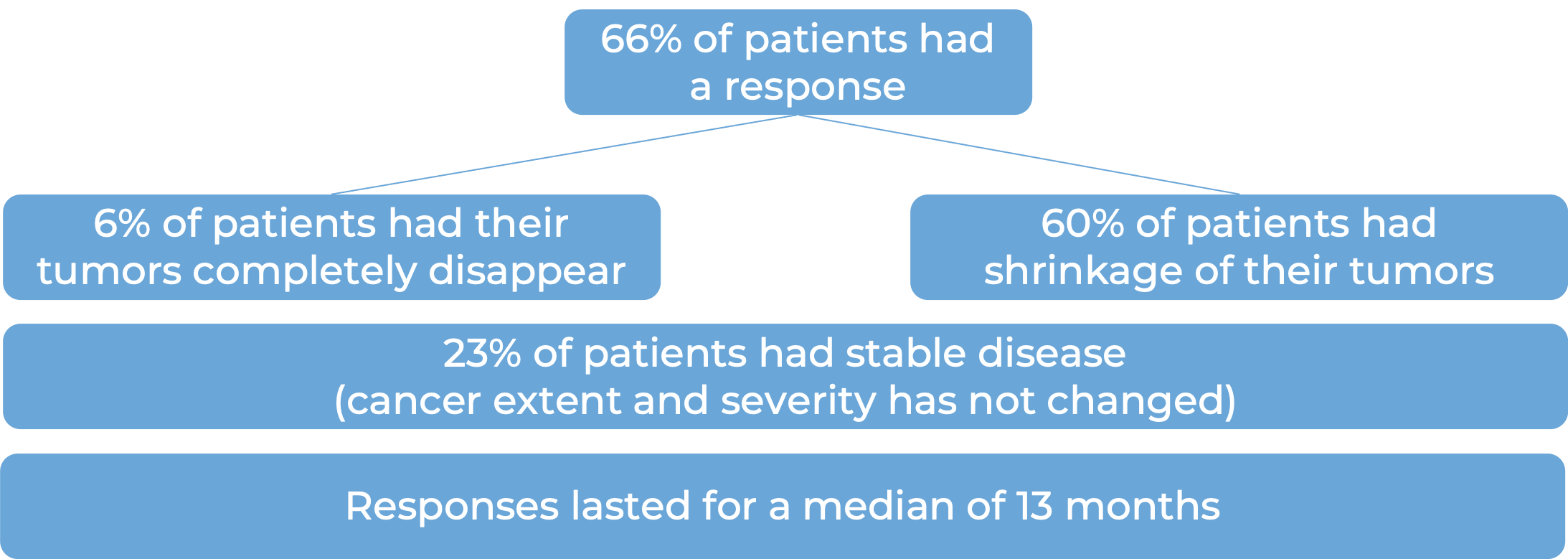
In a clinical study, 821 patients with previously treated advanced kidney cancer were treated with either Opdivo or everolimus (an oral chemotherapy):
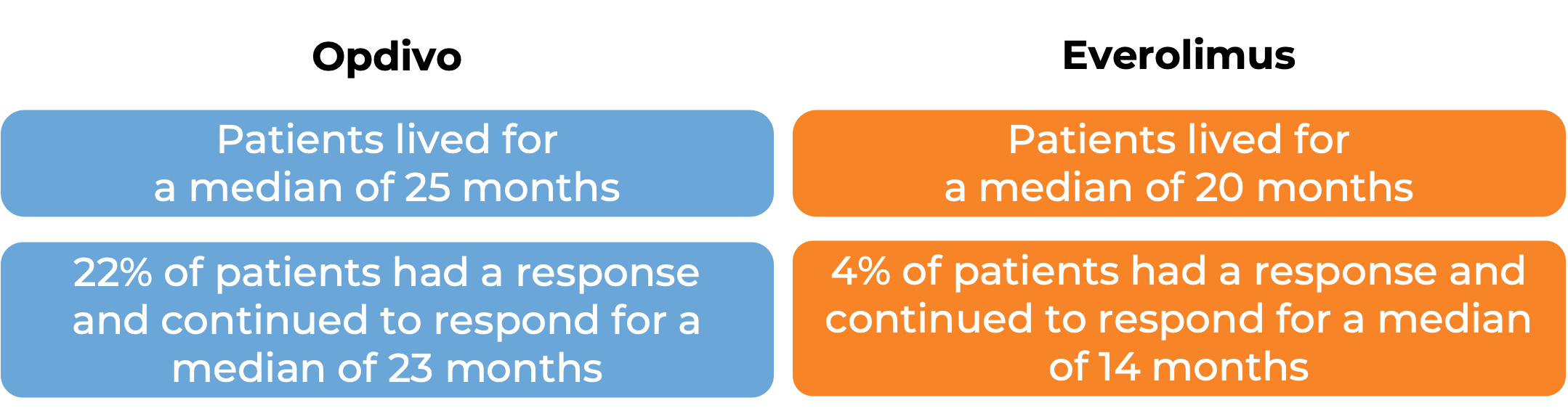
(For a definition of median click HERE.)
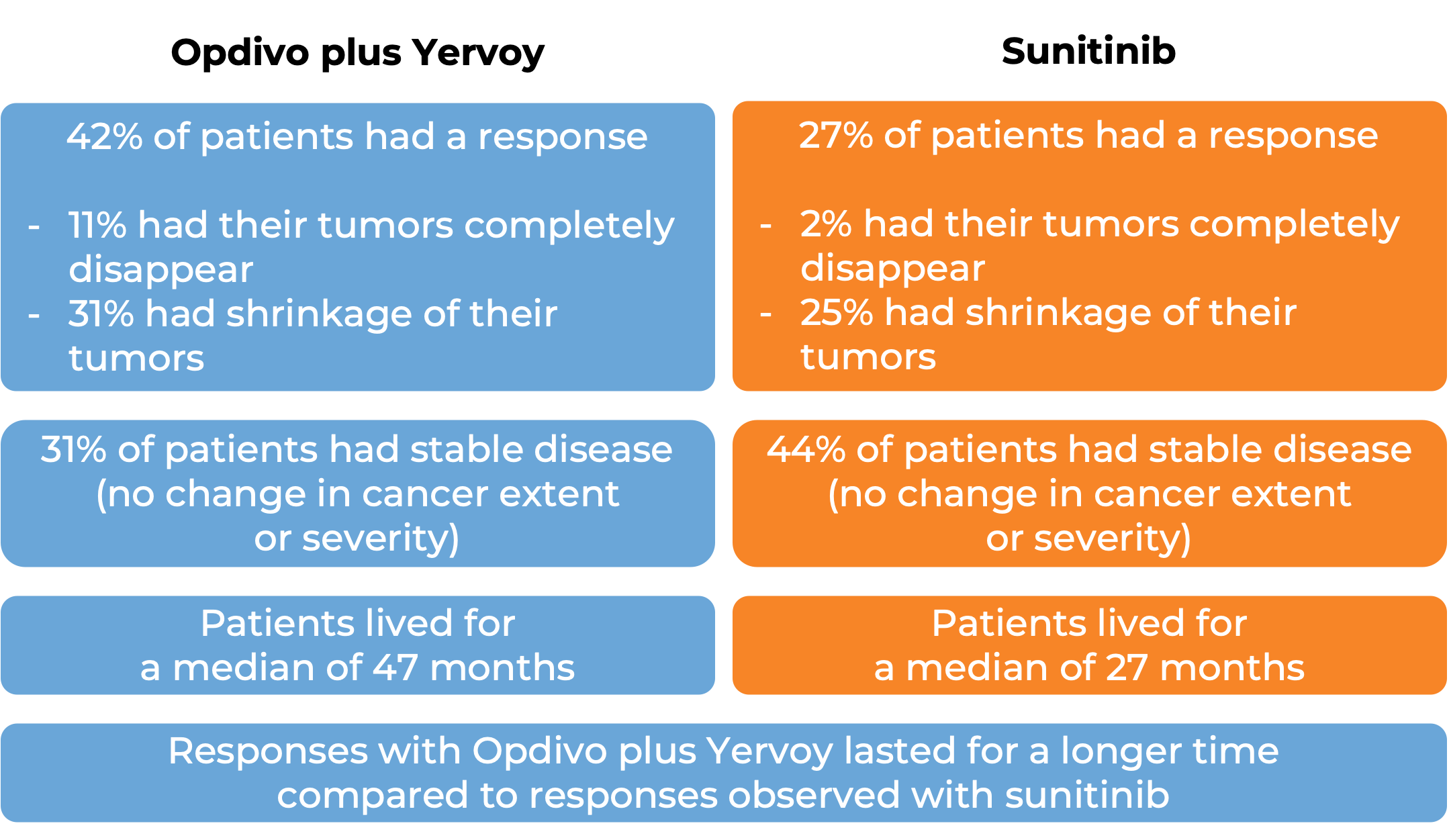
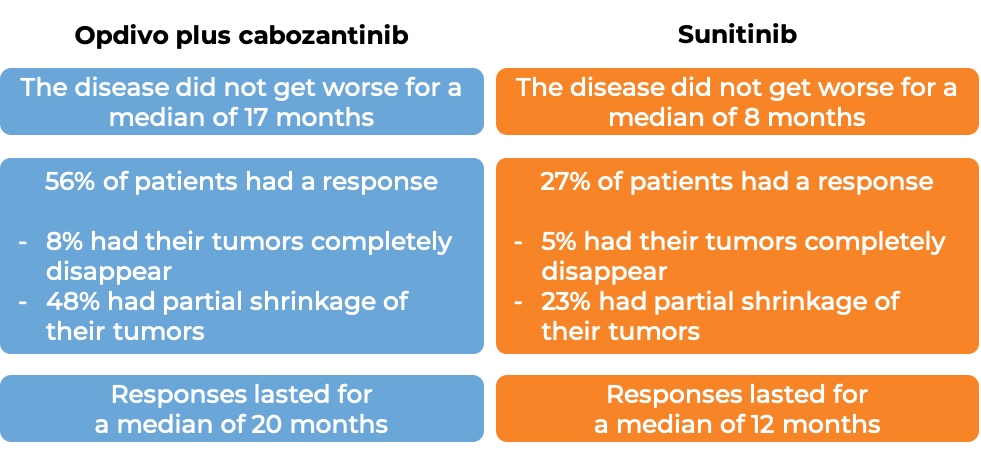
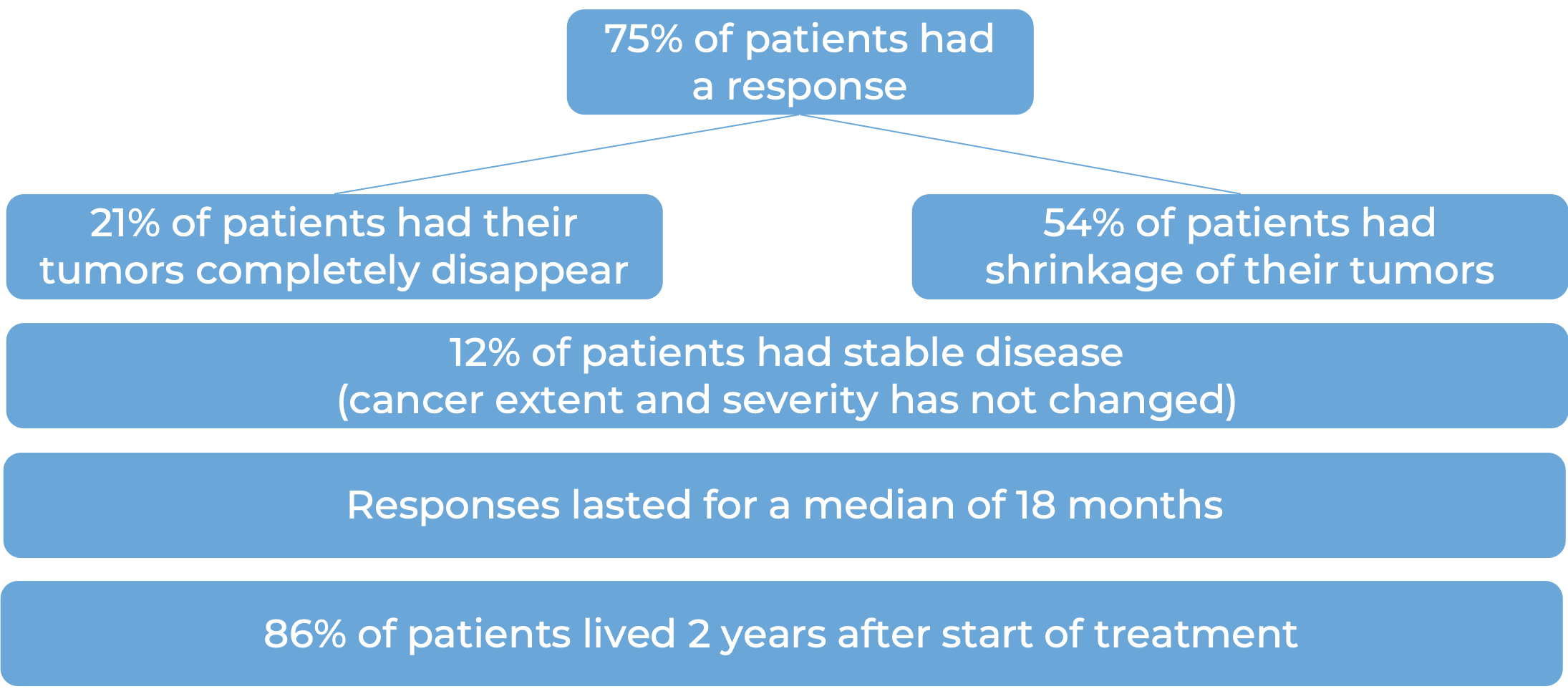
In a clinical study, 651 patients with advanced renal cell carcinoma who had not received any other treatment for their advanced disease, were treated with either Opdivo and cabozantinib or sunitinib (an angiogenesis inhibitor). At at median follow-up of 18 months (and extended median follow-up of 44 months for survival):
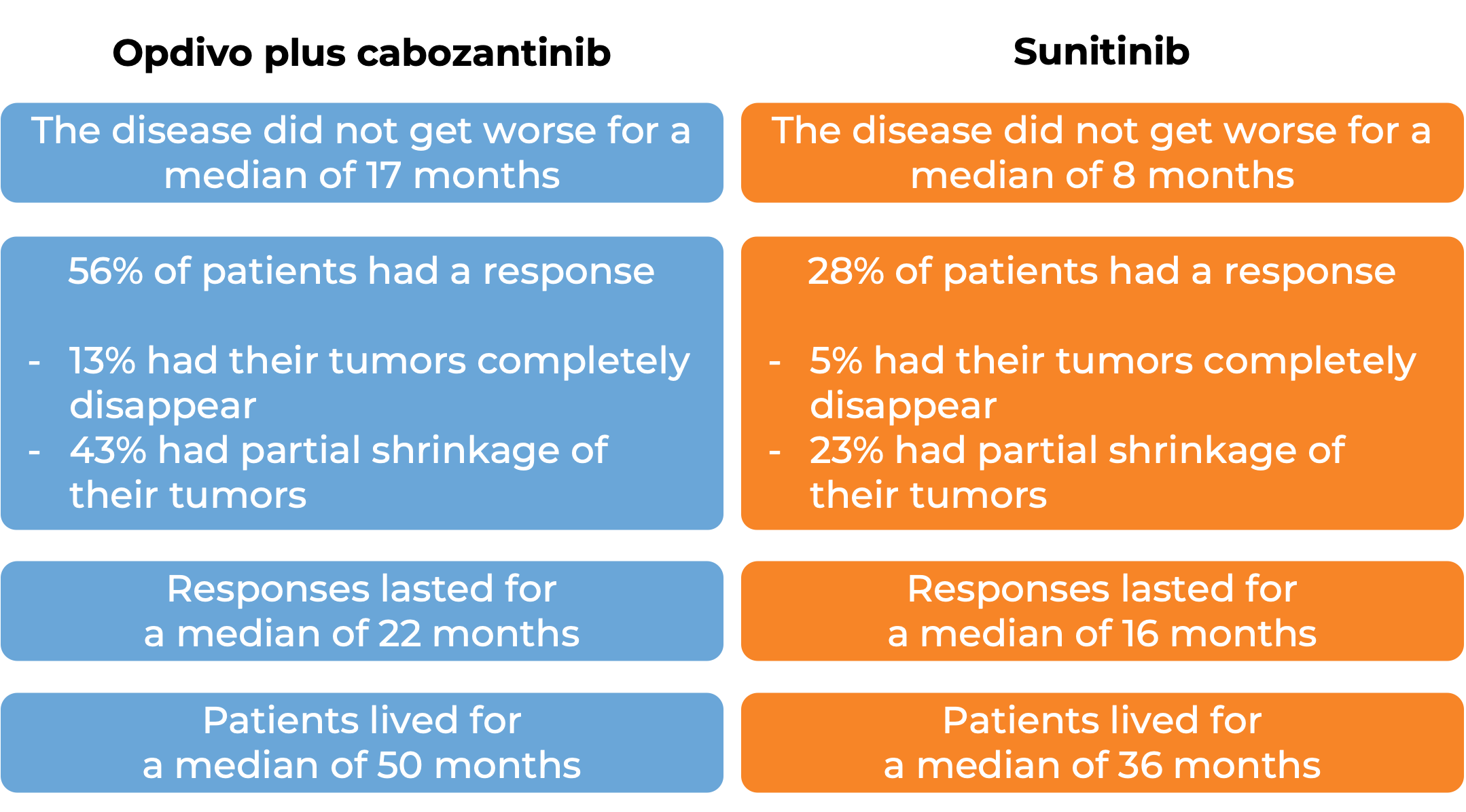
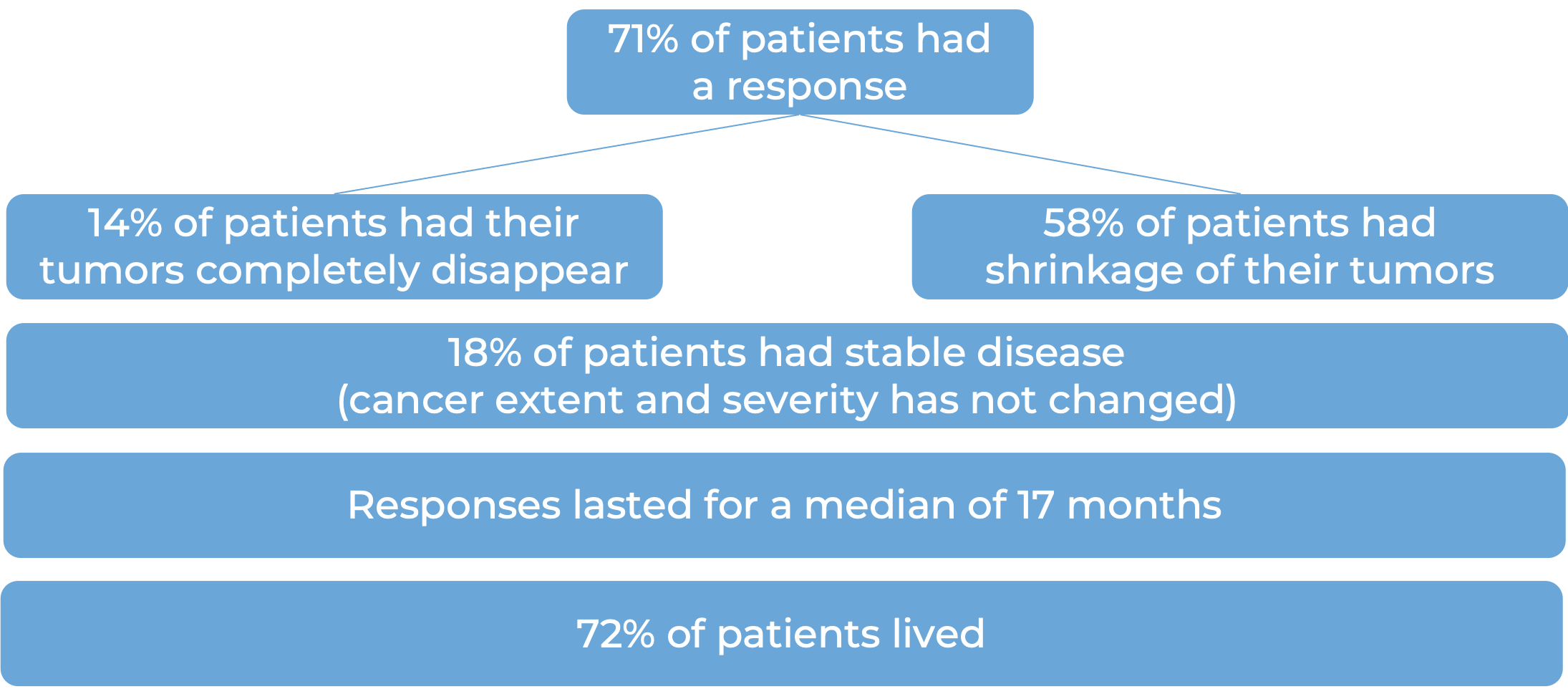
In a clinical study, 847 patients with previously untreated advanced kidney cancer were treated with either a combination of Opdivo and ipilimumab (Yervoy)or with sunitinib (an oral chemotherapy):
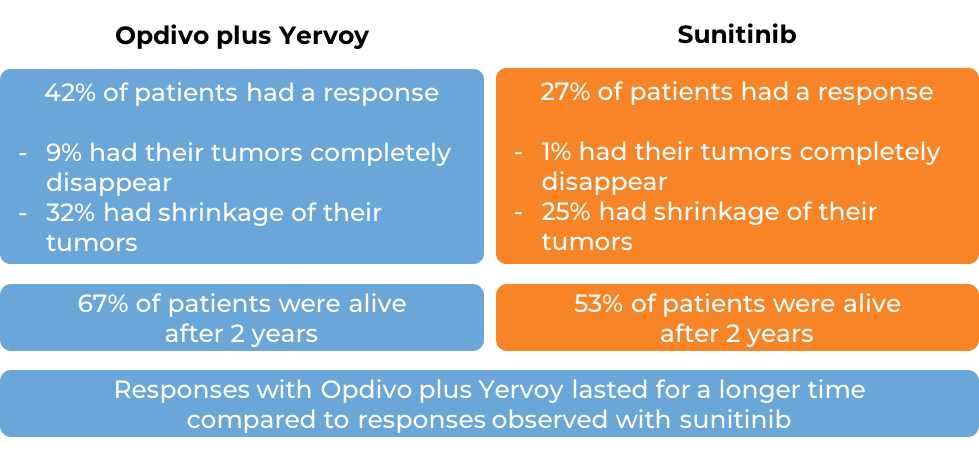
Classical Hodgkin lymphoma
In two clinical studies, a total of 95 patients with classical Hodgkin lymphoma who had received brentuximab vedotin (Adcetris) following autologous stem cell transplant, and the disease had come back or gotten worse were treated with Opdivo. At a median follow-up of 15 months:
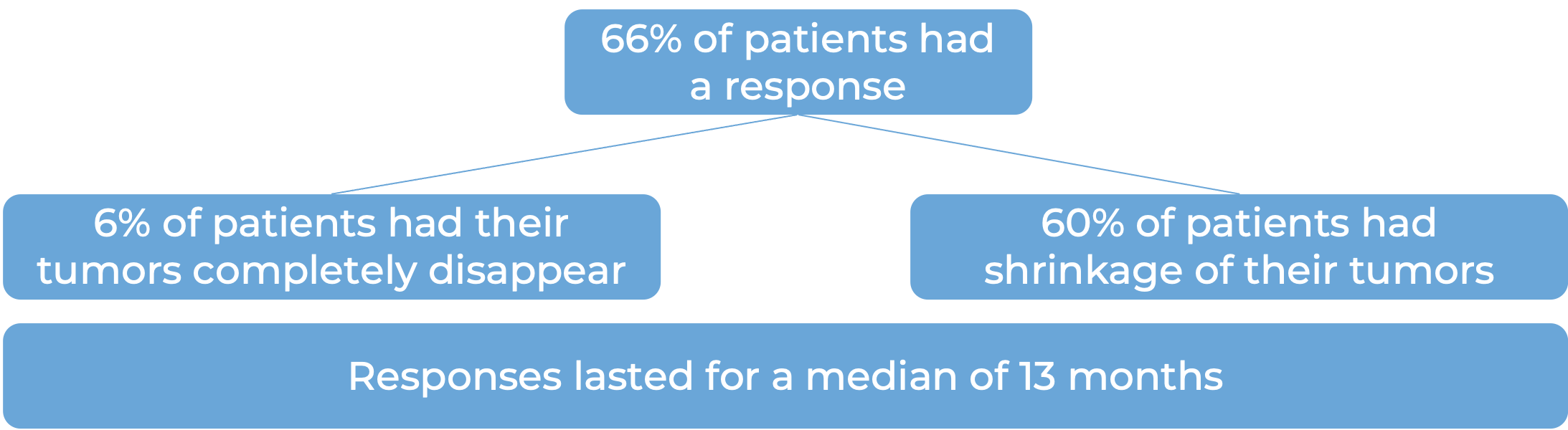
In the same two clinical trials, a total of 258 patients with classical Hodgkin lymphoma who had received brentuximab vedotin (Adcetris) before, after, or before and after autologous stem cell transplant, and the disease had come back or gotten worse were treated with Opdivo.
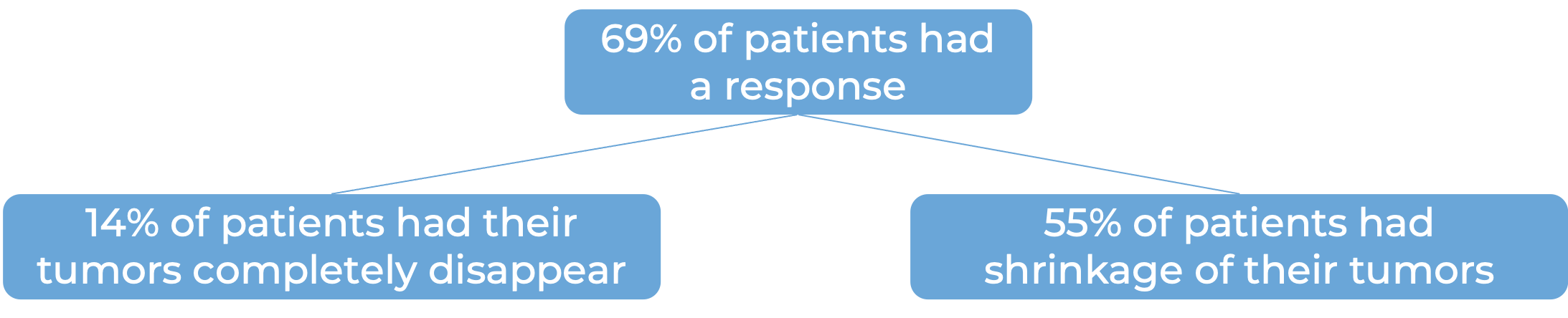
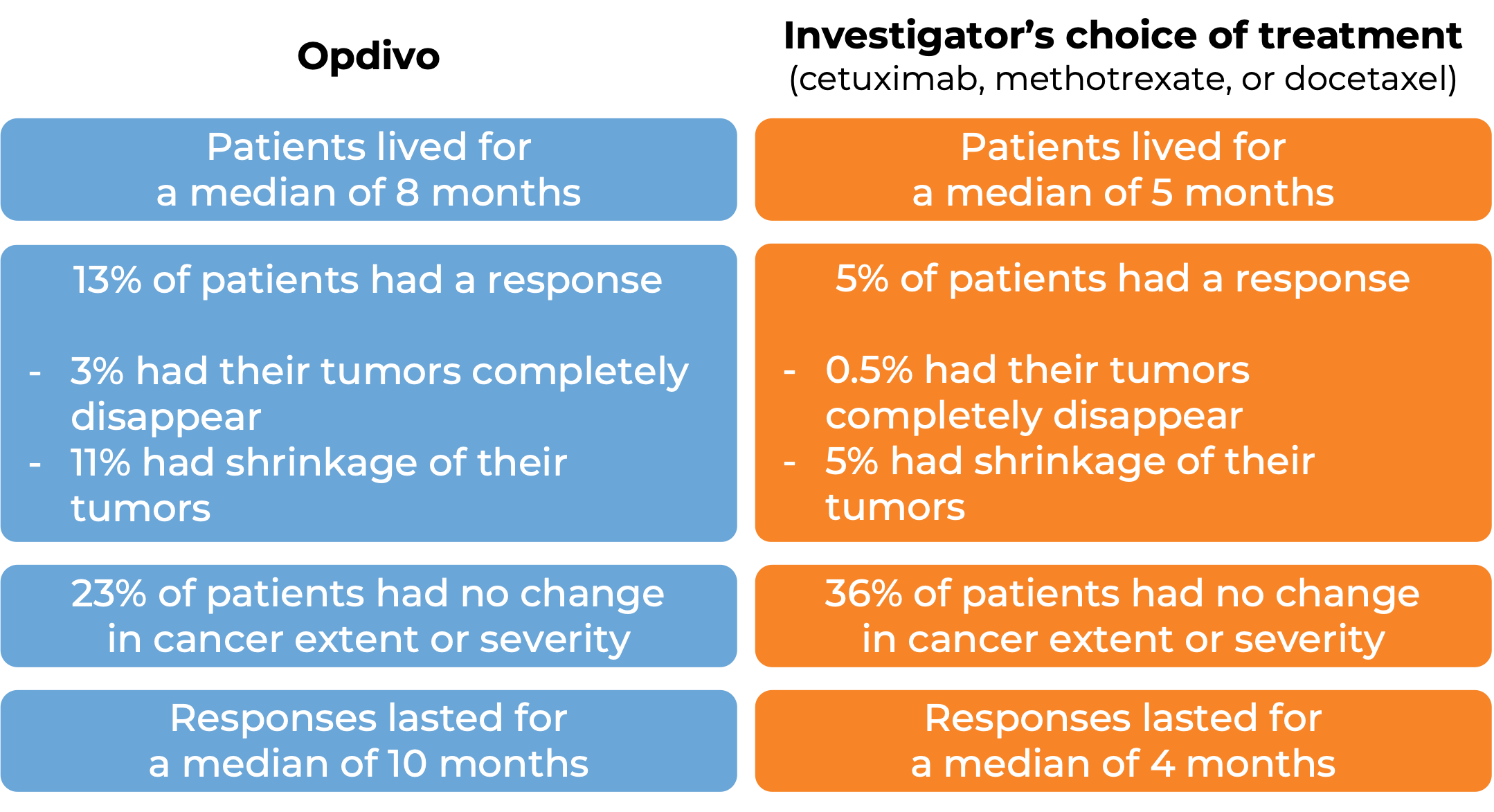
Head and neck squamous cell cancer
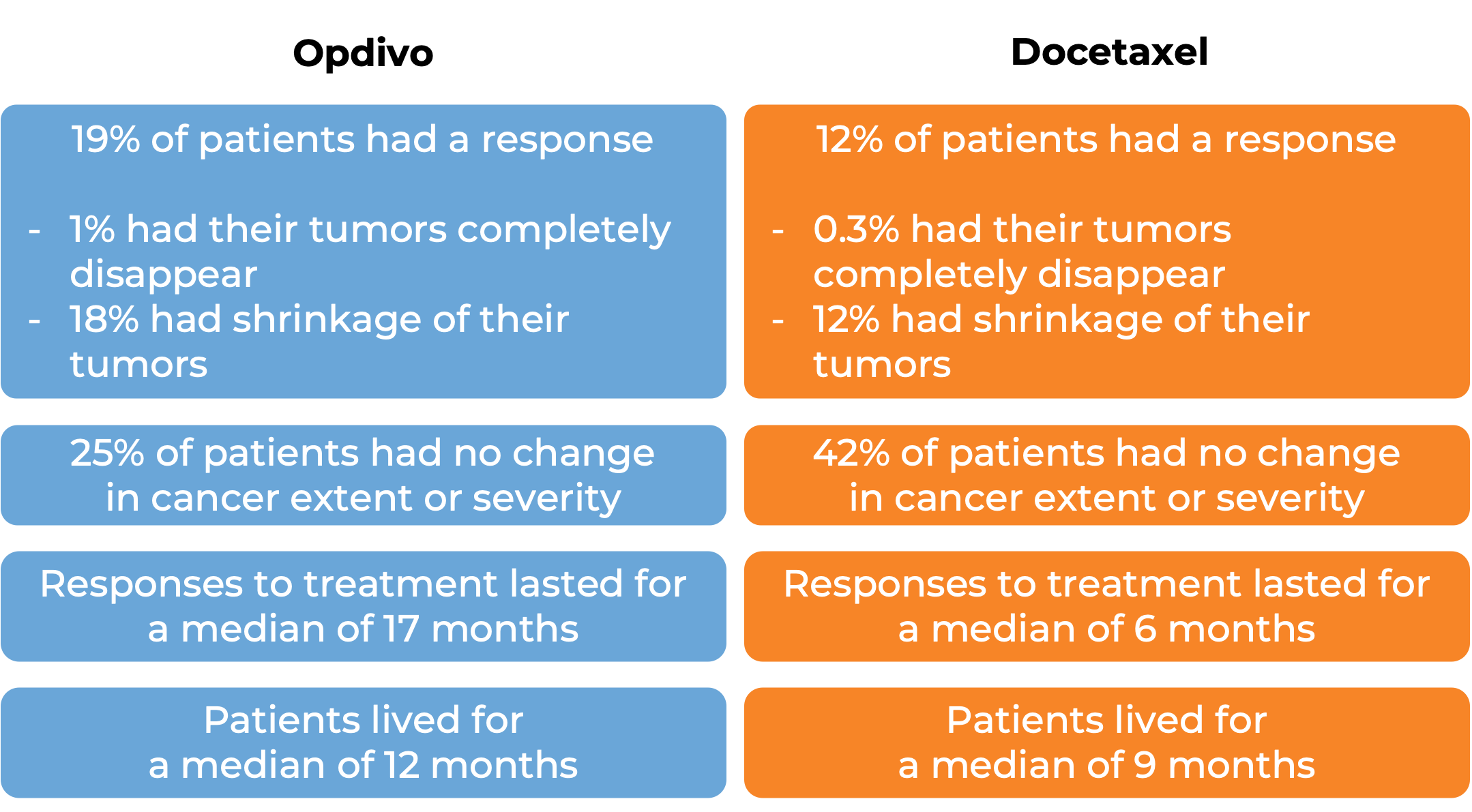
In a clinical study, 361 patients with head and neck squamous cell cancer were treated with Opdivo or with the investigator’s choice of treatment (cetuximab, methotrexate, or docetaxel):
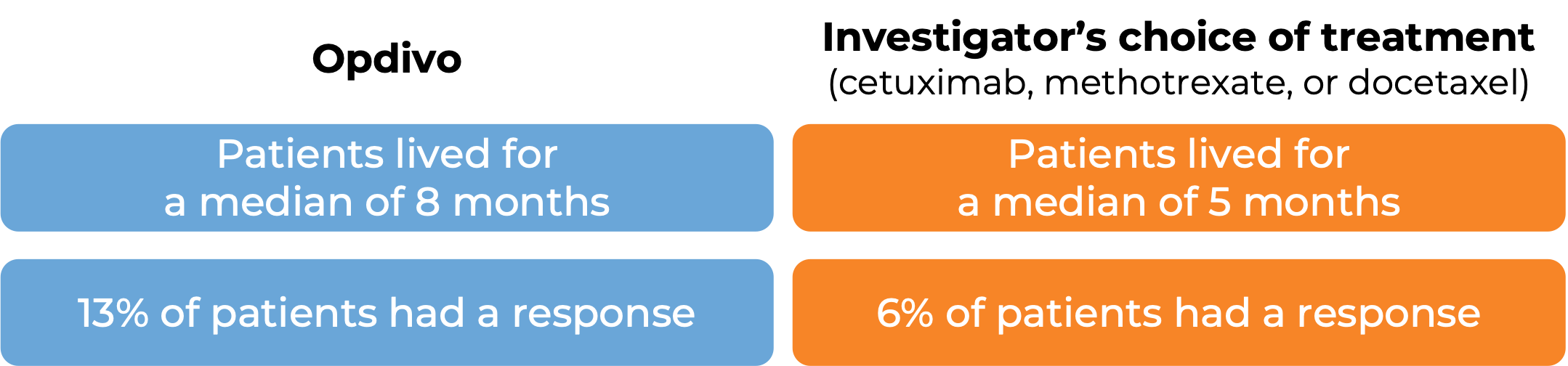
(For a definition of median click HERE.)
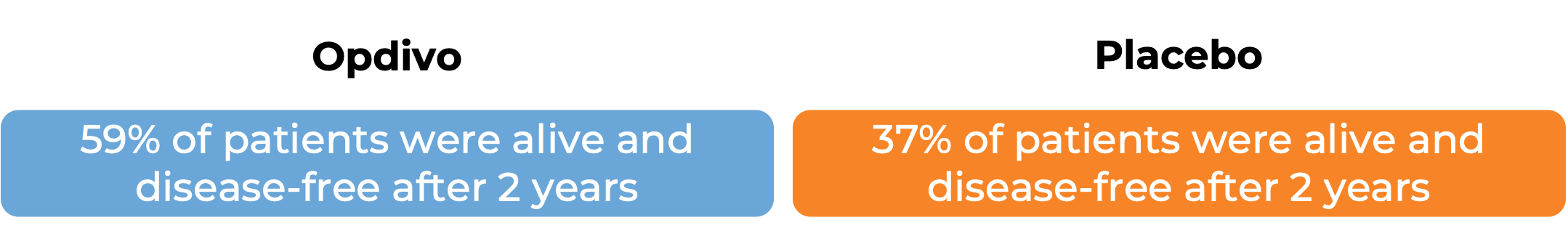
Advanced bladder and urinary tract cancer
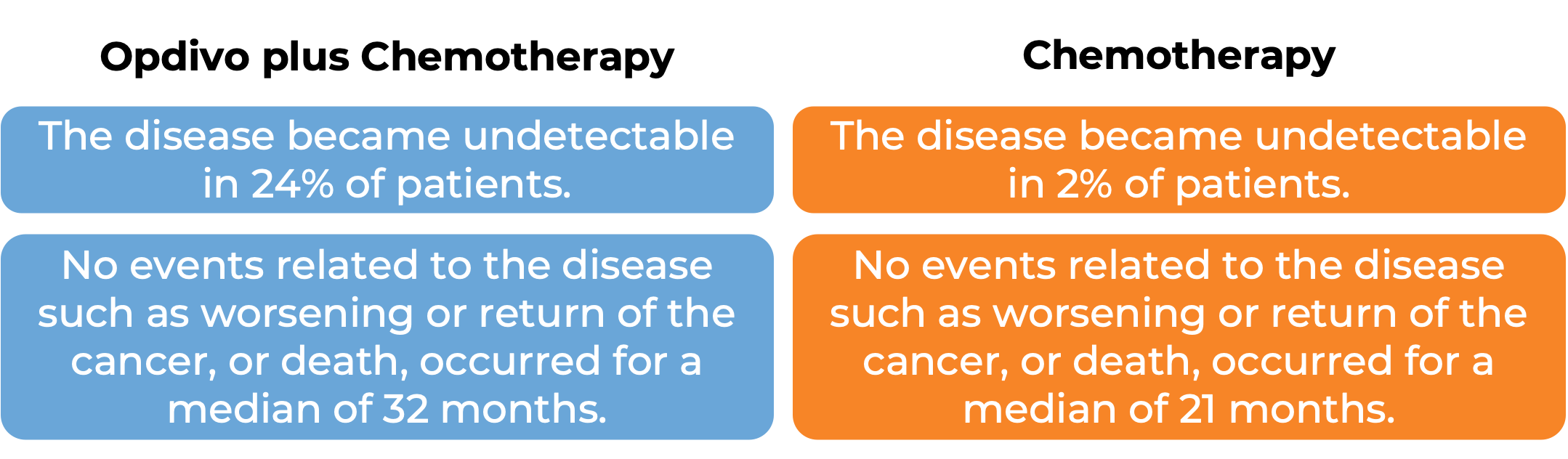
In a clinical study, 608 patients with urothelial carcinoma (the most common type of bladder and urinary tract cancer) that could not be removed by surgery or had spread to other parts of the body, and who had not received treatment for their advanced disease, were treated with Opdivo plus cisplatin and gemcitabine chemotherapy or chemotherapy alone. At a median follow-up of 34 months:
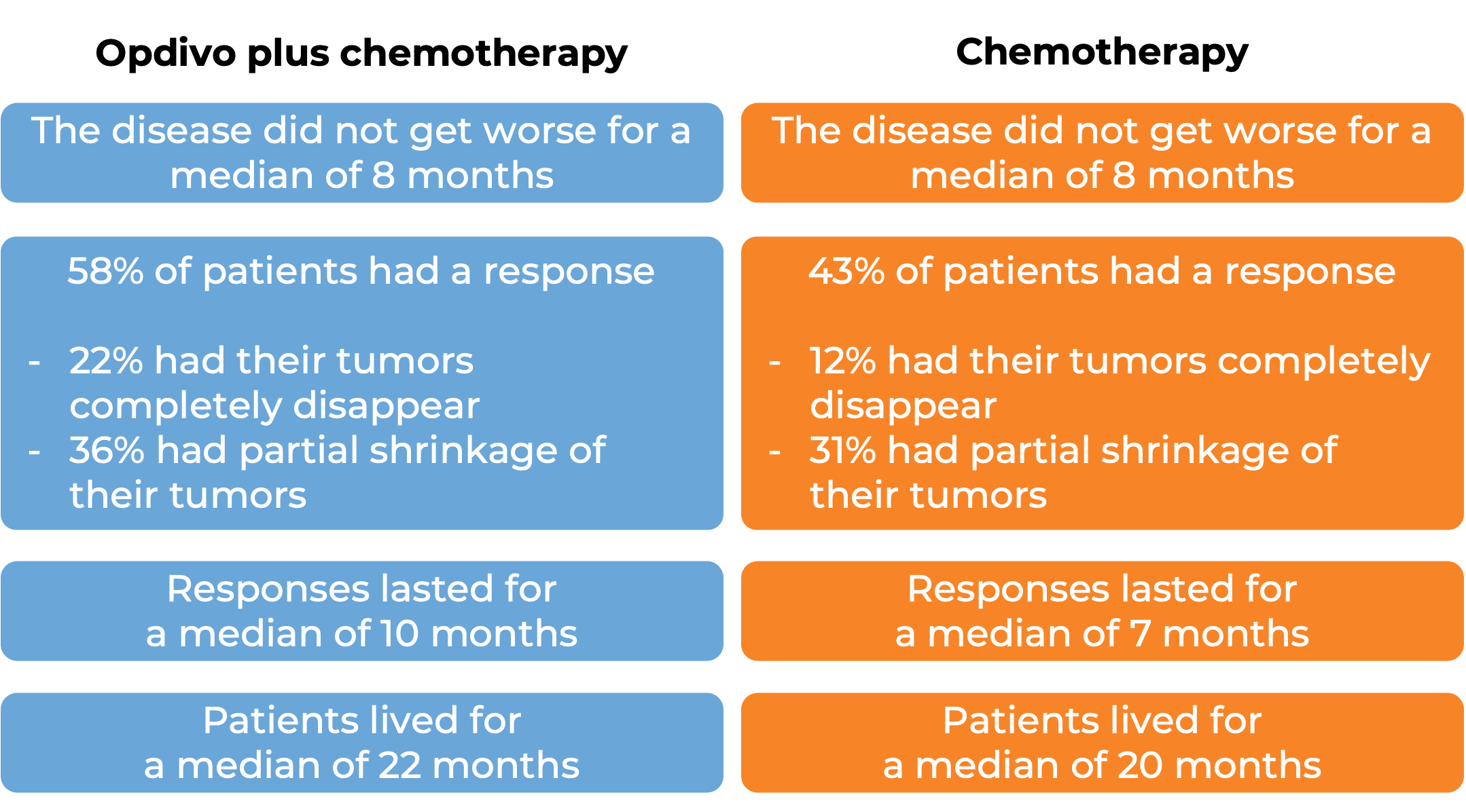
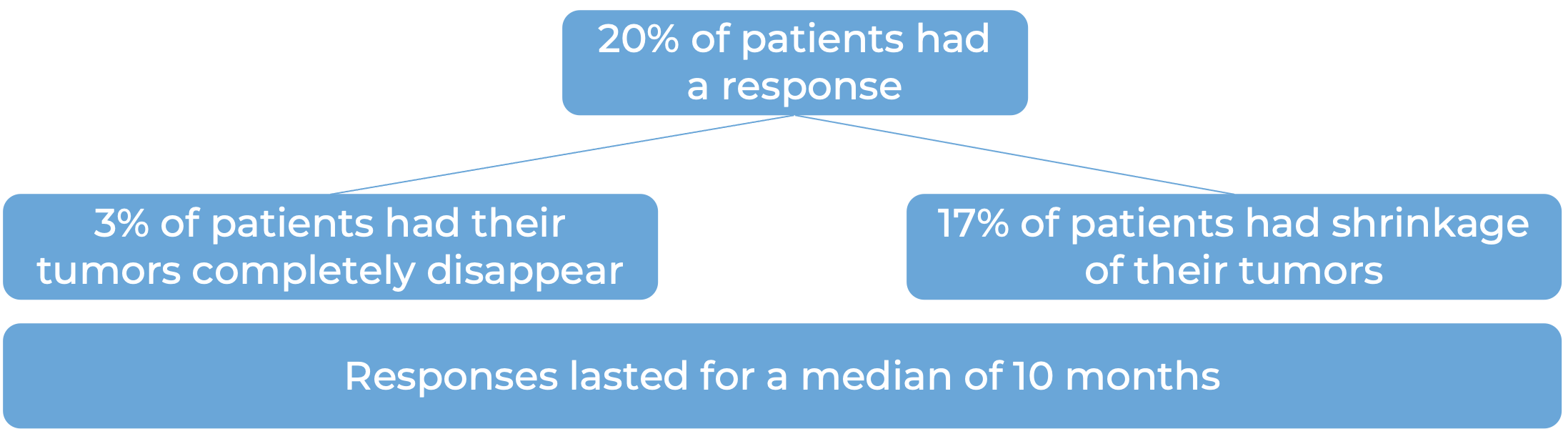
In another clinical study, 270 patients with advanced urothelial carcinoma (the most common type of bladder and urinary tract cancer) that had grown or spread, who had been treated with chemotherapy containing platinum, and it did not work or stopped working, were treated with Opdivo:
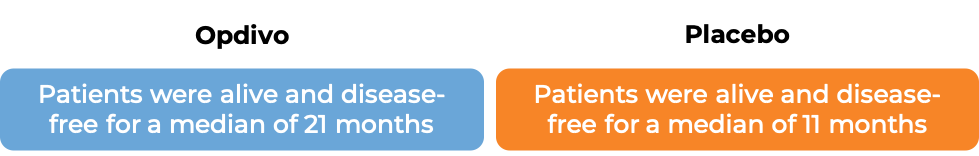
In another clinical study, 709 patients with advanced bladder cancer whose cancer had been removed by surgery, but who were at a high risk of coming back, were treated with Opdivo or placebo. At a median follow-up of 20 to 21 months:
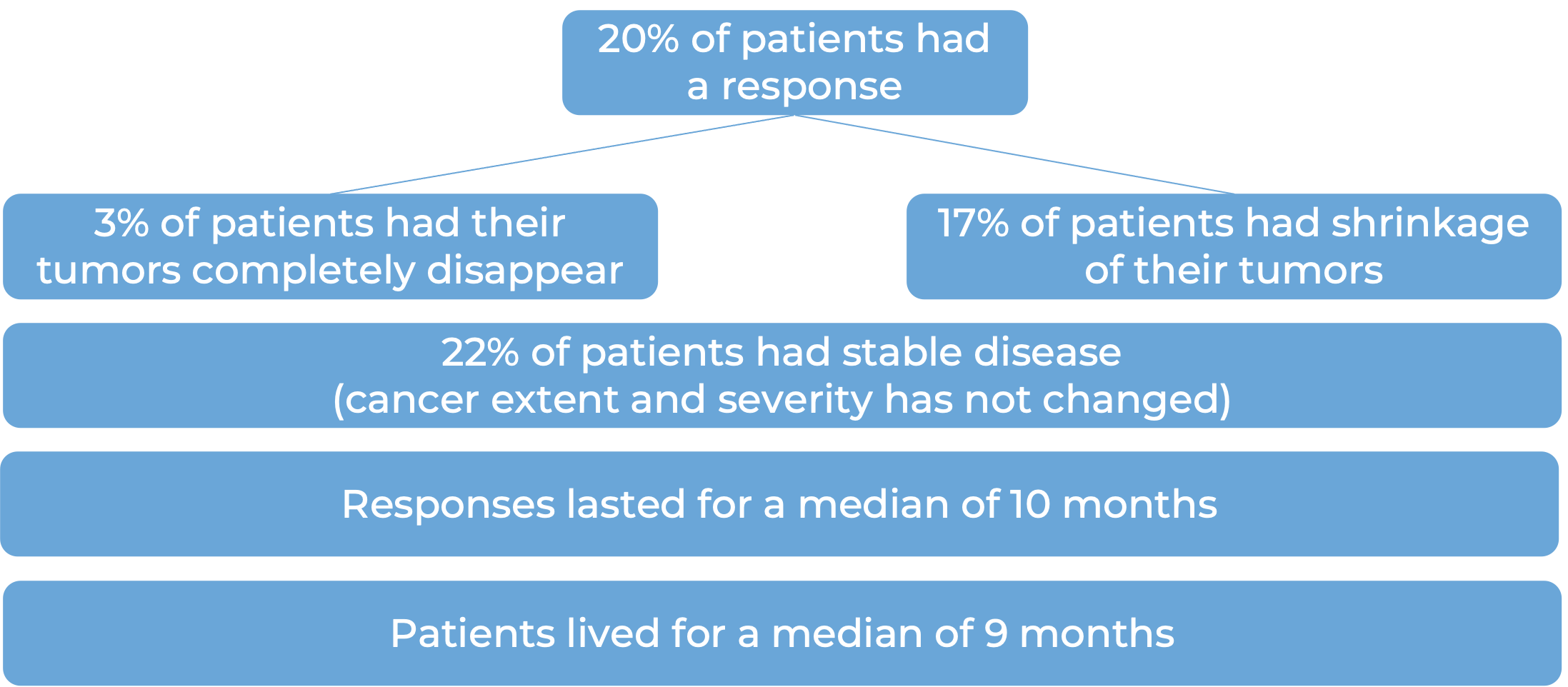
Advanced colorectal cancer (MSI-H/dMMR)
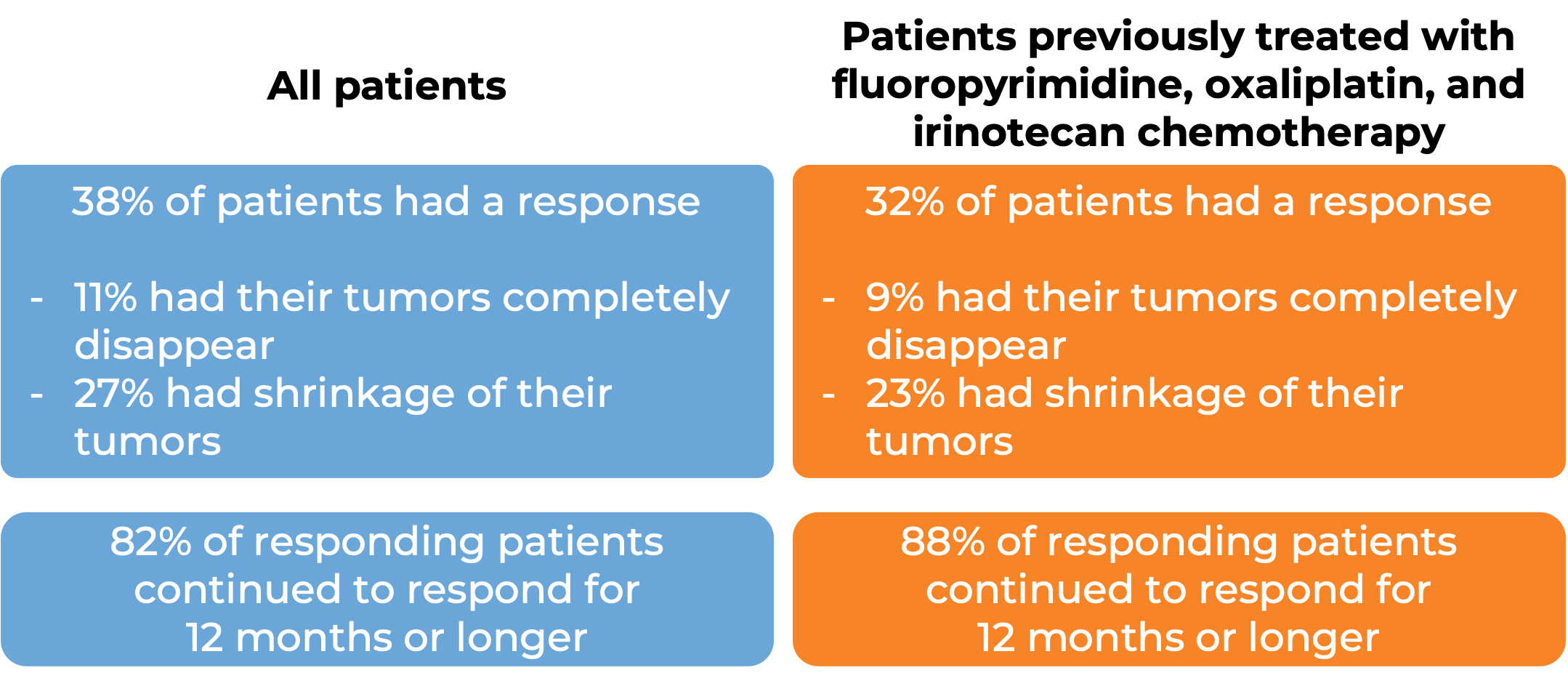
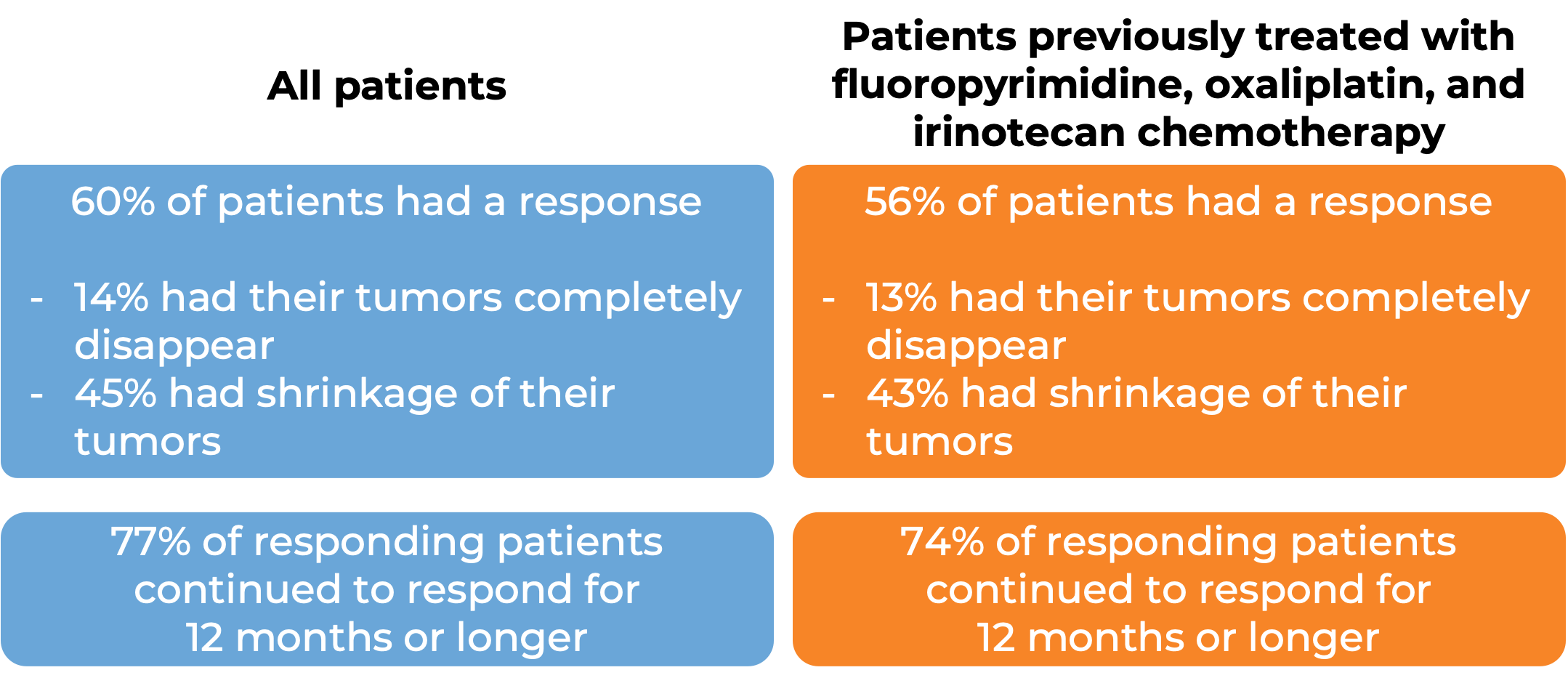
In a clinical study, 193 patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) colon or rectal (“colorectal”) cancer (including patients who have been treated with a fluoropyrimidine, oxaliplatin, and irinotecan chemotherapy, and the treatment did not work or stopped working) were treated with Opdivo or a combination of Opdivo and ipilimumab (Yervoy).
At a median follow-up of 34 months among patients treated with Opdivo:
 At a median follow-up of 28 months, among patients treated with Opdivo and ipilimumab (Yervoy):
At a median follow-up of 28 months, among patients treated with Opdivo and ipilimumab (Yervoy):
Advanced liver cancer
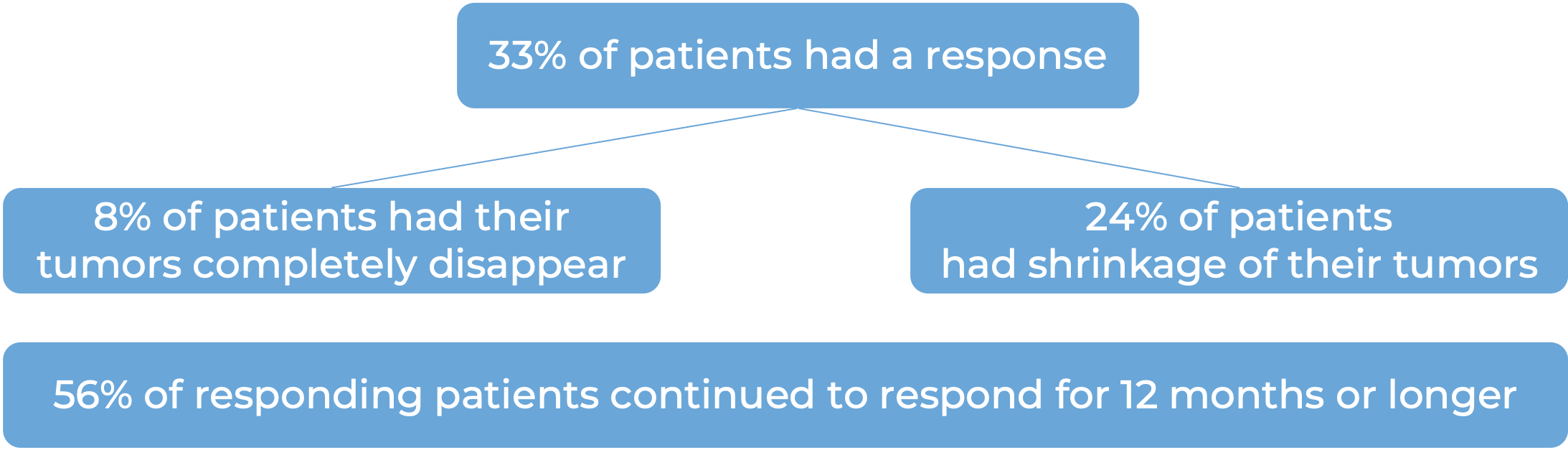
In a clinical study, 49 patients with advanced liver cancer who had been treated with sorafenib (Nexavar), and it either did not work, stopped working, or was not tolerated, were treated with Opdivo plus ipilimumab (Yervoy). At a minimum follow-up of 28 months:
Advanced esophageal squamous cell carcinoma
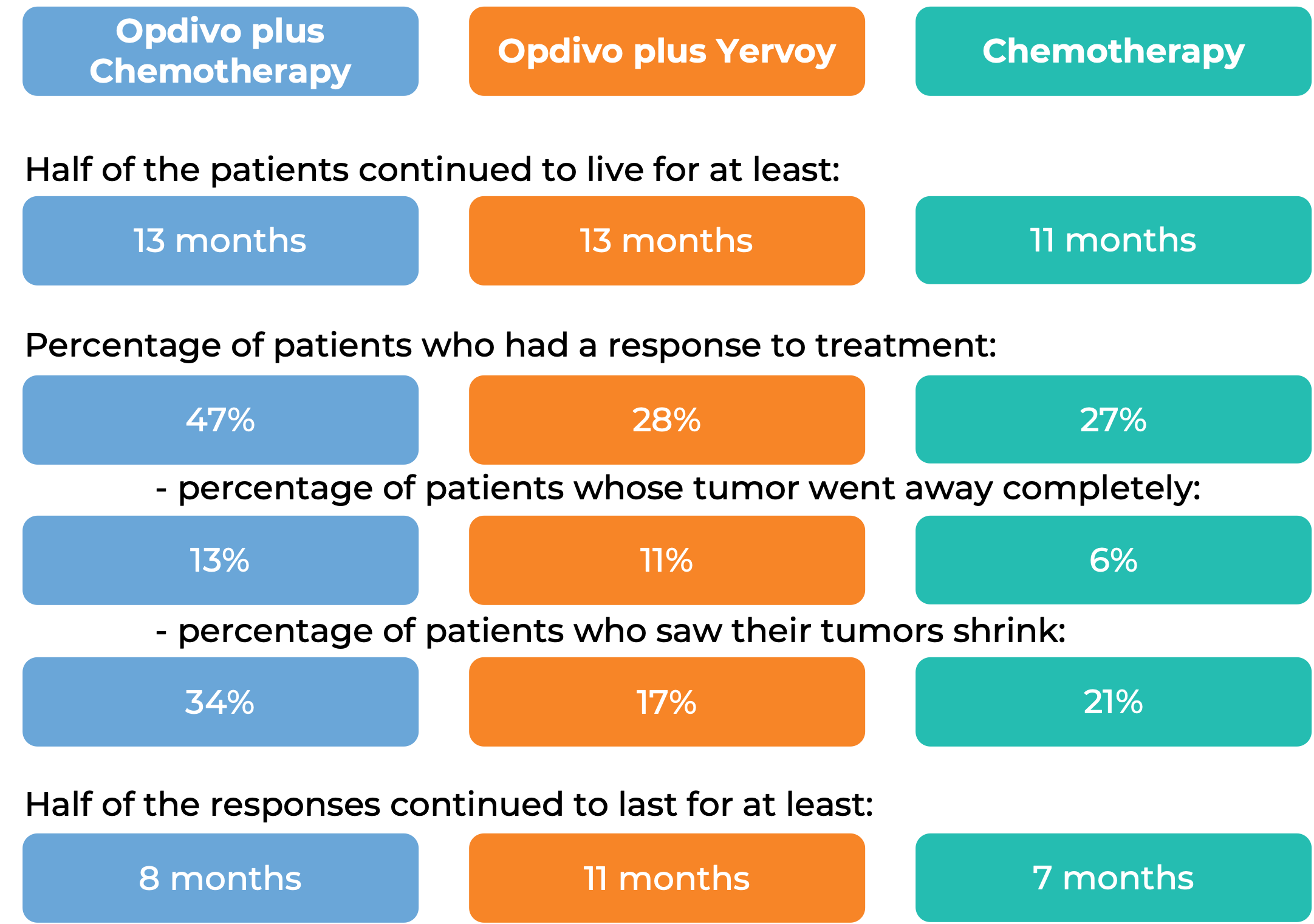
In a clinical study, 970 patients with previously untreated esophageal squamous cell carcinoma that could not be removed by surgery, recurred after surgery, or had spread to other parts of the body, were treated with either:
- Opdivo plus chemotherapy (fluorouracil and cisplatin),
- Opdivo plus ipilimumab (Yervoy), OR
- chemotherapy alone.
At a minimum follow-up of 13 months, among all patients:
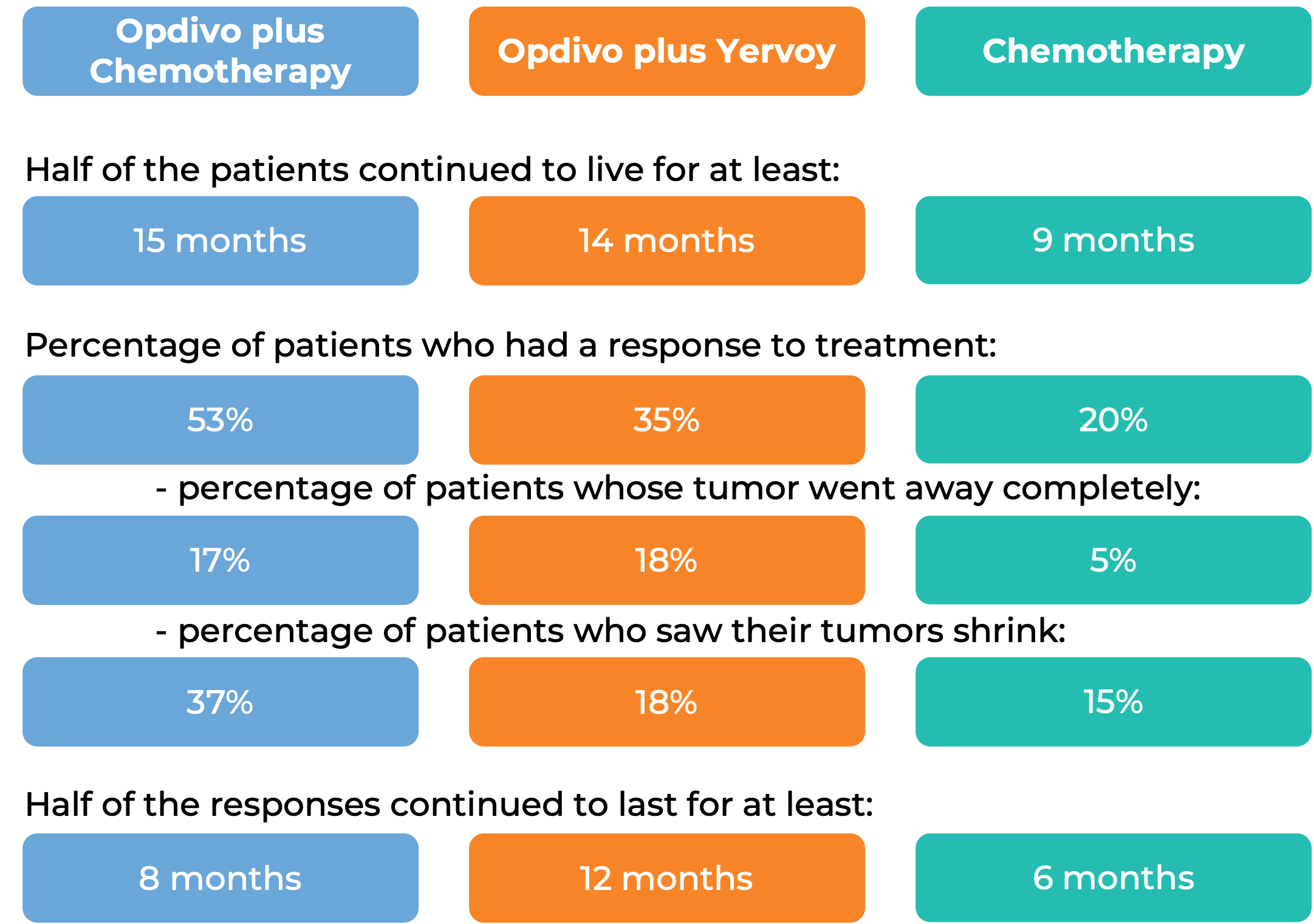
At a minimum follow-up of 13 months, among 473 patients whose tumors tested positive for the PD-L1 molecule:
In another clinical study, 419 patients with esophageal squamous cell carcinoma whose cancer had spread or come back and could not be removed by surgery, and who had been treated with chemotherapy containing fluoropyrimidine and platinum, but did not respond to or could not tolerate this treatment, were treated with either Opdivo or investigator’s choice of chemotherapy (docetaxel or paclitaxel). At a minimum follow-up of 18 months:
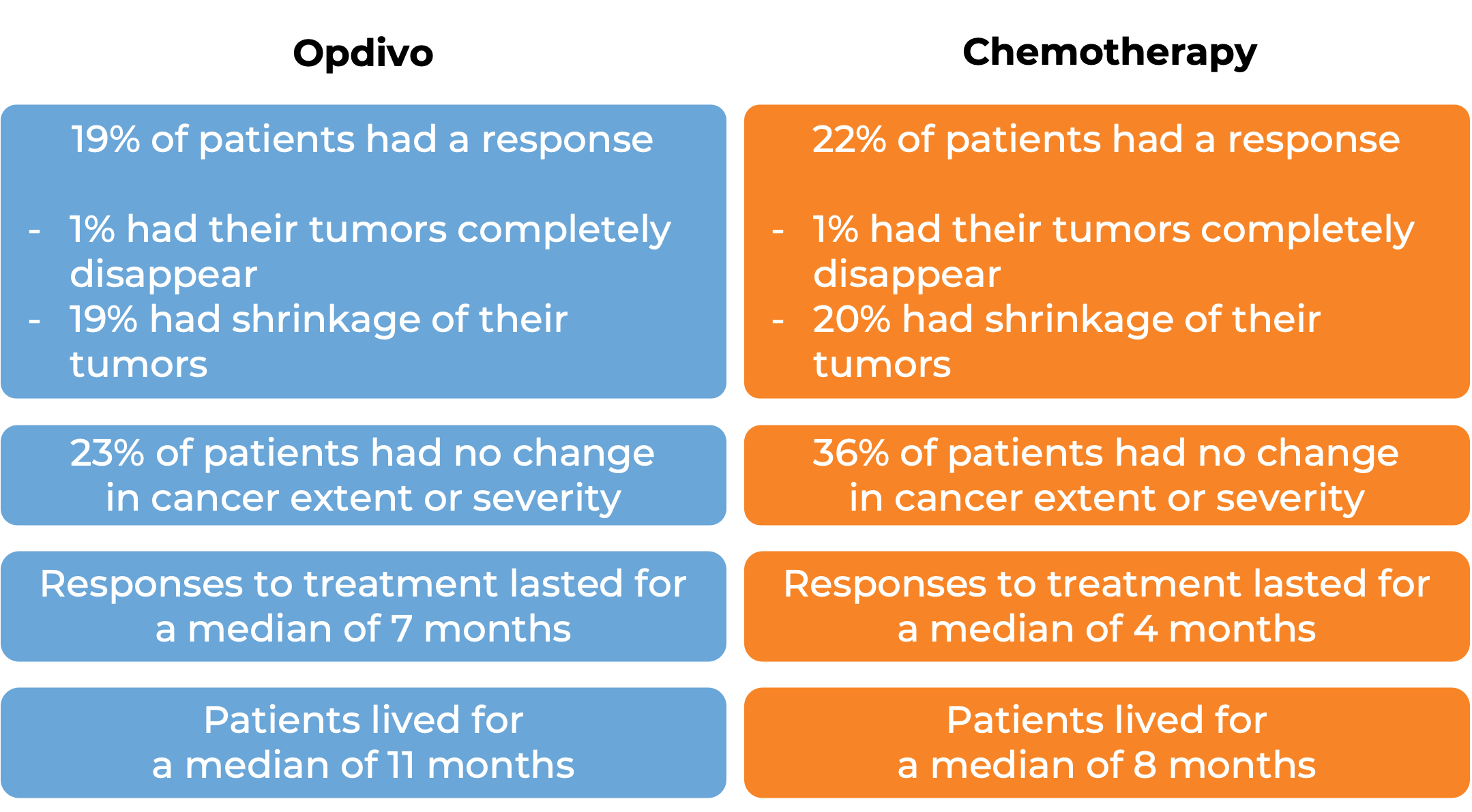
(For the definition of “median”, click HERE.)
In another clinical study, 794 patients with esophageal or gastroesophageal junction cancer that had been treated with chemotherapy and concurrent radiation and was then removed by surgery, but who still had cancer cells remaining in their body after treatment, were treated with either Opdivo or a placebo.
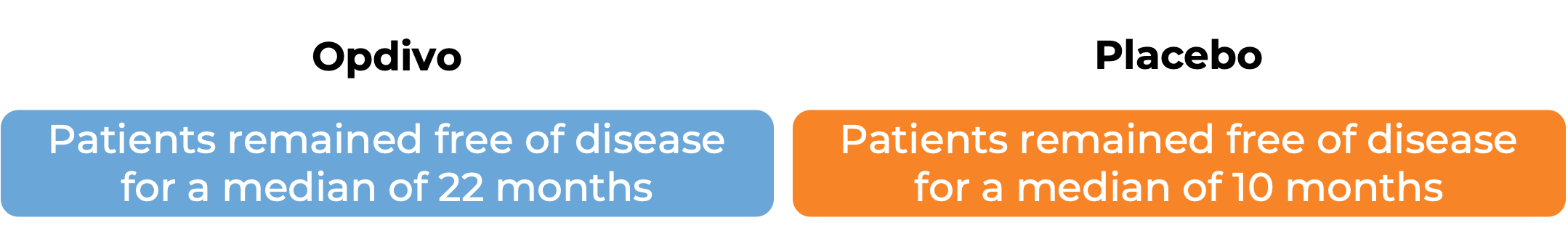
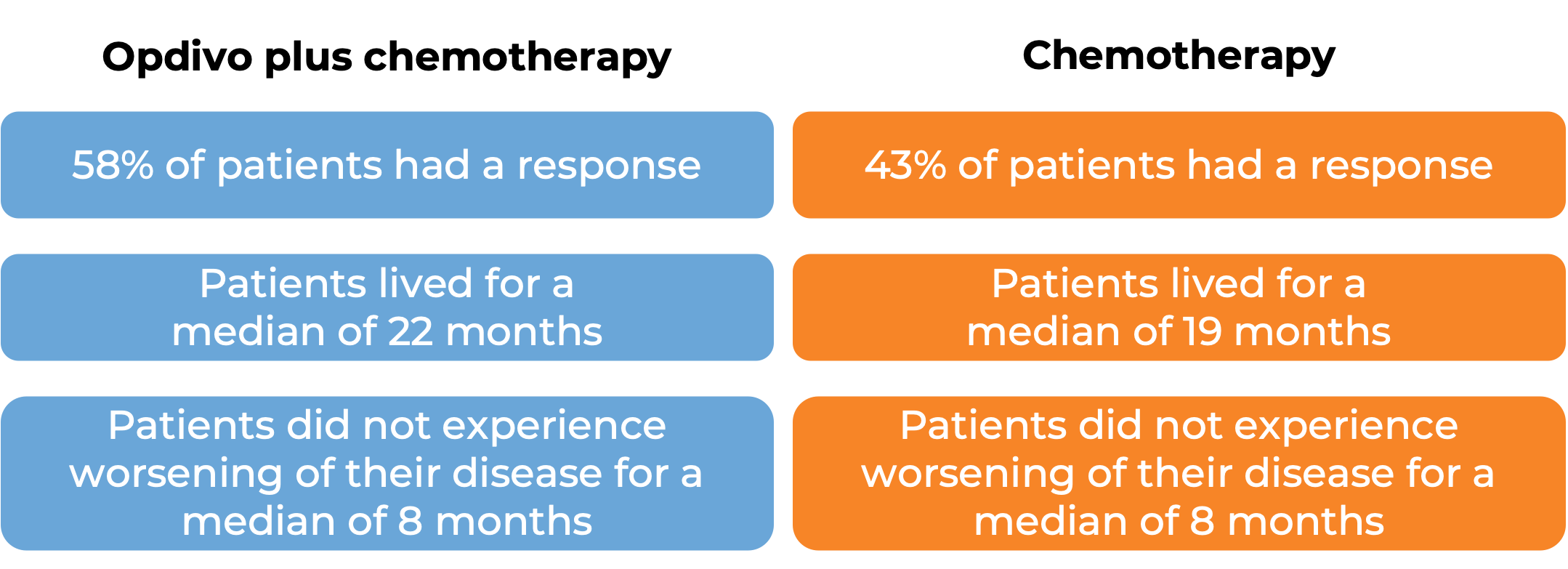
Advanced stomach cancer
In a clinical trial, 1581 patients with previously untreated advanced stomach (gastric), gastroesophageal junction, or esophageal adenocarcinoma, were treated with either Opdivo in combination with chemotherapy (mFOLFOX6 [fluorouracil, leucovorin, and oxaliplatin] or CapeOX [capecitabine and oxaliplatin]) or chemotherapy alone. At a minimum follow-up of 12 months:
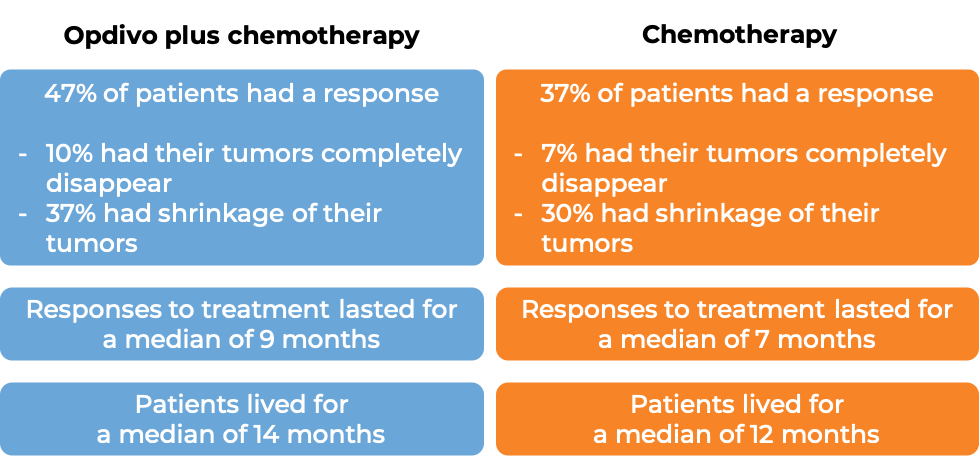
(For the definition of "median" click HERE.)
Malignant pleural mesothelioma
In a clinical study of 605 patients with newly diagnosed malignant pleural mesothelioma whose cancer could not be removed by surgery, patients were treated with either Opdivo in combination with ipilimumab (Yervoy) for up to 2 years or chemotherapy (cisplatin or carboplatin plus pemetrexed). At a minimum follow-up of 22 months:
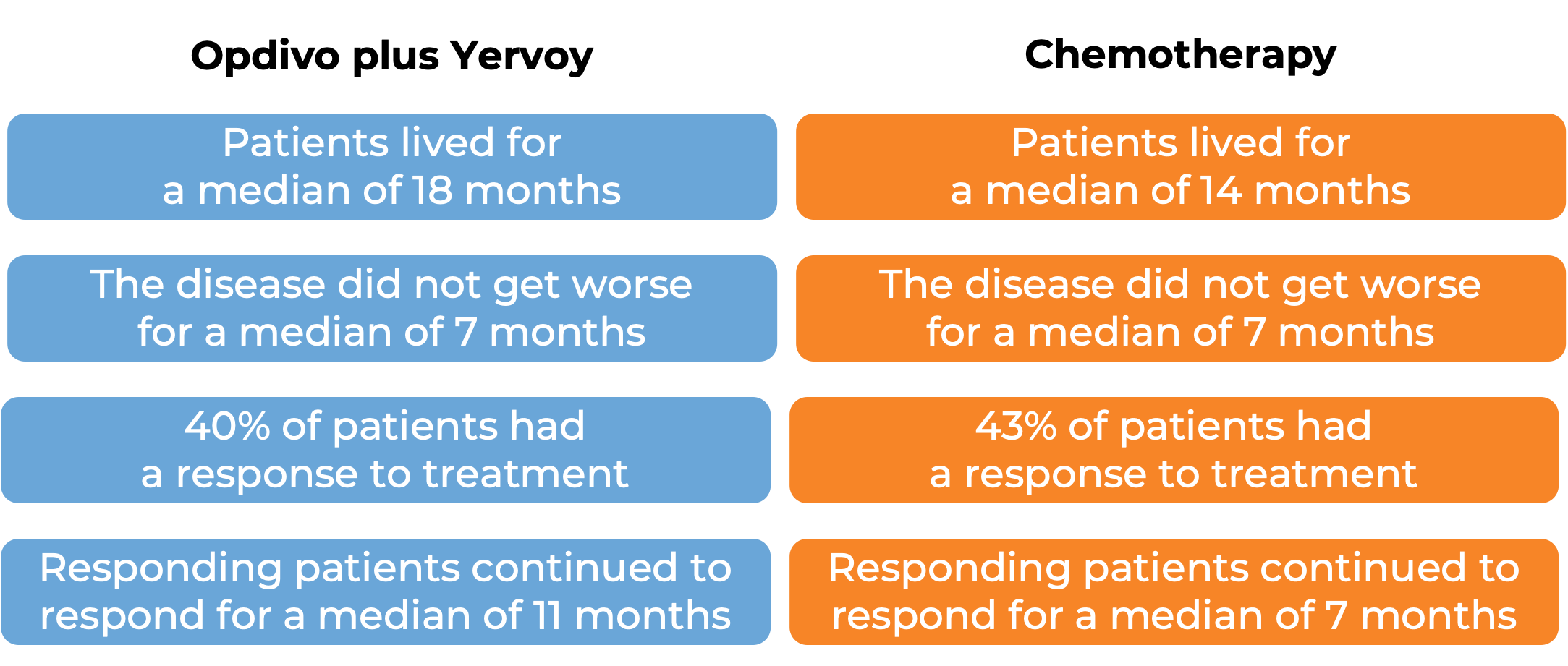
(For a definition of median click HERE.)
What are the side effects?
The most common side effects of Opdivo include fatigue, rash, pain (in the muscles, bones, joints, back, and stomach area), headache, itching, diarrhea, constipation, nausea, vomiting, decreased appetite, cough, shortness of breath, upper respiratory tract or urinary tract infections, and fever.
Opdivo can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Opdivo can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Opdivo include inflammation of the lungs, liver, brain, kidneys (which can lead to kidney failure), or colon (which can result in tears or holes in the intestine). Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Opdivo may cause Type 1 diabetes. Skin rash (which could become severe and life-threatening), and reactions related to the infusion may also occur. Patients should report any symptoms to their healthcare provider who can then initiate actions to limit or reverse the side effects.
Patients may experience other side effects when Opdivo is used in combination with other treatments. For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Bristol‑Myers Squibb
Approval
FDA and EMA (but different indications, this document is for FDA only)
Links to drug websites
Other references
- Nivolumab monotherapy in patients with advanced platinum-resistant urothelial carcinoma: Efficacy and safety update from CheckMate 275. Siefker-Radtke AO, Baron AD, et al. Journal of Clinical Oncology (2019).
- https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-approves-opdivo-nivolumab-ye-0
- https://news.bms.com/news/details/2020/Opdivo-nivolumab-Plus-Yervoy-ipilimumab-Demonstrates-Durable-Survival-Benefit-vs.-Chemotherapy-in-Patients-with-Previously-Untreated-Malignant-Pleural-Mesothelioma/default.aspx
- https://ascopost.com/news/september-2020/checkmate-9er-trial-shows-benefit-of-nivolumabcabozantinib-for-advanced-kidney-cancer/
- Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. Bajorin DF, et al. The New England Journal of Medicine (2021)
- Nivolumab in Previously Untreated Melanoma without BRAF Mutation. Robert C, et al. The New England Journal of Medicine (2015)
- Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. Doki Y, et al. The New England Journal of Medicine (2022)
- Adjuvant nivolumab in resected stage IIB/C melanoma: primary results from the randomized phase 3 CheckMate 76K trial. Kirkwood JM, et al. Nature Medicine (2023)
- Adjuvant Nivolumab versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results from CheckMate 238. Larkin J, et al. Clinical Cancer Research
- Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. Van der Heijden MS, et al. The New England Journal of Medicine (2023)
- Nivolumab plus cabozantinib versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended follow-up from the phase III randomised CheckMate 9ER trial. Powles T, et al. ESMO Open (2024)
Last updated: September 12, 2024