How is this drug name pronounced?
Tebentafusp: teh-BEN-tah-fusp
Kimmtrak: KIM-trak
What cancer(s) does this drug treat?
Kimmtrak can only be used to treat patients who test positive for HLA-A*02:01.
Advanced uveal melanoma
Kimmtrak is approved for:
- Patients with uveal melanoma (a type of eye cancer) that has spread to other parts of the body from the original cancer site, or cannot be removed by surgery.
Limitations of use
Age: The safety and efficacy of Kimmtrak in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Kimmtrak may cause harm to a fetus, and is not recommended for use during pregnancy. Women should not start Kimmtrak treatment when pregnant and are advised to use contraception during treatment and for at least 1 week after the last dose of Kimmtrak. The risks associated with Kimmtrak during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for 1 week after the last dose of Kimmtrak.
What type of immunotherapy is this?
- Bispecific antibody therapy
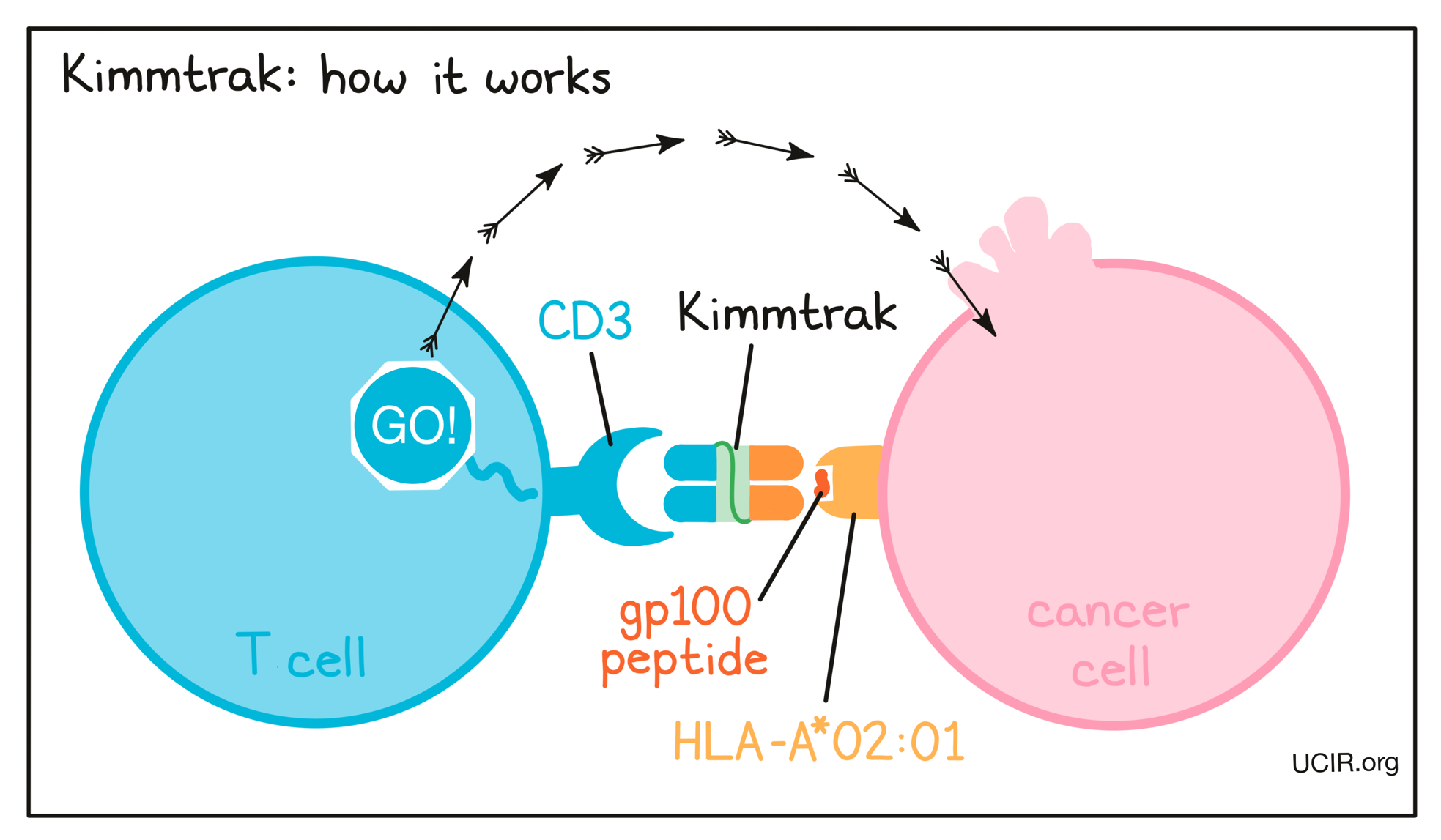
How does this drug work?
Target:
- CD3 receptor on T cells
- gp100 peptide-HLA-A*02:01
Kimmtrak is a “bispecific protein” that was artificially made in the laboratory and can very precisely bind two different target molecules at the same time. Kimmtrak binds to:
- a molecule called CD3 on the surface of a T cell – the primary immune cell involved in killing cancer cells. CD3 is part of the T cell receptor, which is critical to the function of the T cell and is involved in stimulating the T cell to become active.
- a complex of molecules called gp100 peptide–HLA-A*02:01 that is present on the surface of some cancer cells. gp100 is a molecule that is present in higher than normal amounts in cancer cells compared to healthy skin cells and is a tumor-associated antigen. Some cancer cells present gp100 on HLA-A02:01 molecules on their cell surface, and Kimmtrak can bind to this complex of molecules.
Because Kimmtrak can bind to one molecule on cancer cells and another molecule on T cells at the same time, it acts as a bridge and brings the T cell in close contact with the cancer cell. By binding CD3 on the T cell, Kimmtrak also stimulates the T cell to become activated and kill the cancer cell it is bound to. Bispecific proteins that direct T cells to kill cancer cells by binding to both cells at the same time are known as T cell engagers.
How is the drug given to the patient?
Kimmtrak is administered weekly via a tube into a vein (intravenous infusion, or i.v.). During the first three infusions, the dose is slowly increased to the recommended dose. The first three infusions are administered over the course of 15-20 minutes in a hospital setting, and patients are observed for at least 16 hours after the administration of Kimmtrak for any symptoms related to the infusion. Subsequent infusions do not require a hospital stay, and patients are monitored for at least 30 min after the administration of Kimmtrak.
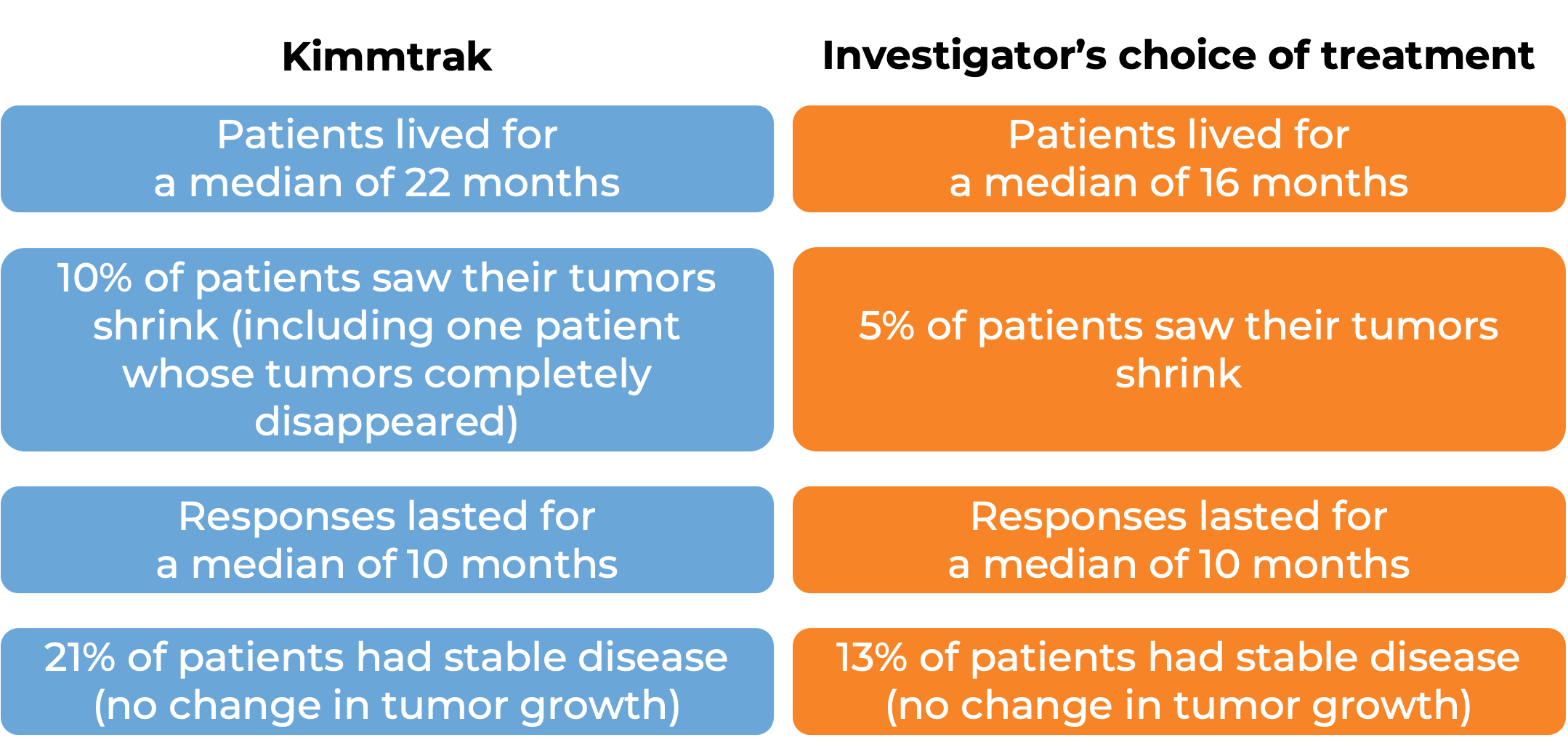
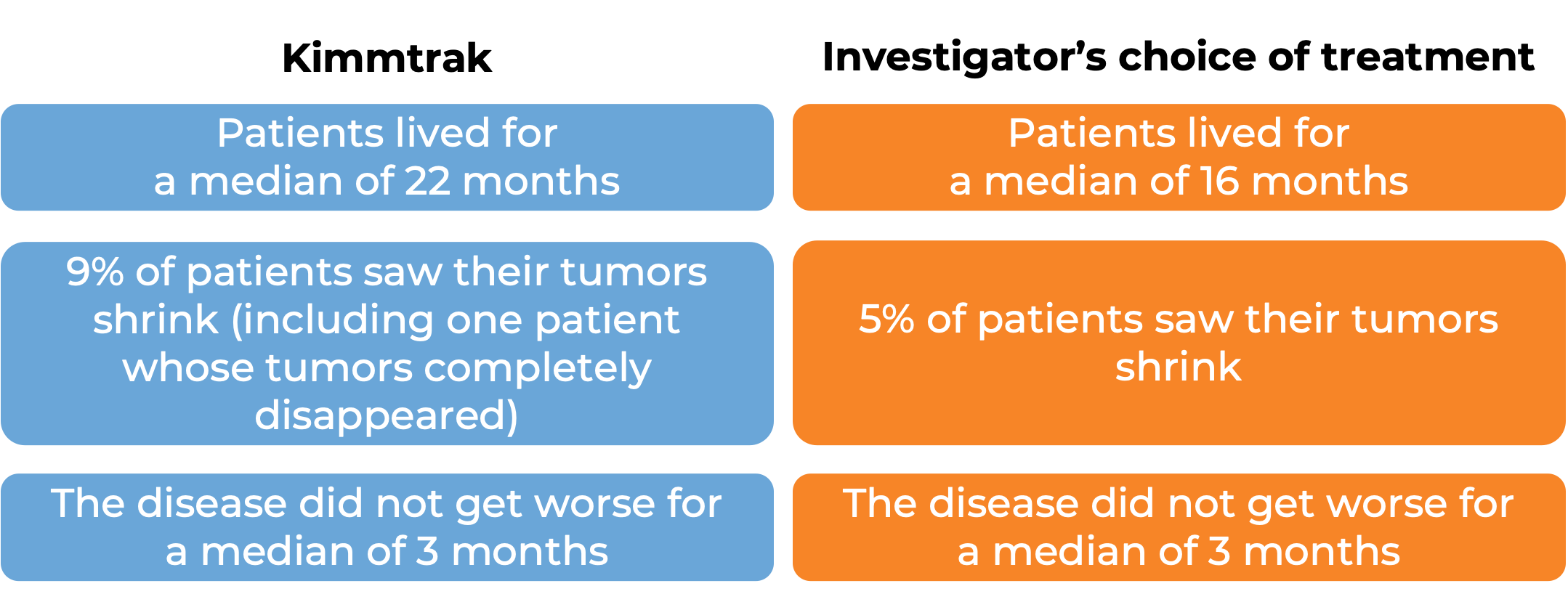
What are the observed clinical results?
It is important to keep in mind that each patient’s outcome is individual and may be different from the results found in the clinical studies. In addition, immunotherapy can sometimes take several months to yield an observable treatment response.
Advanced uveal melanoma
In a clinical trial, 378 patients with uveal melanoma (a type of eye cancer) that had spread to other parts of the body from the original cancer site, or could not be removed by surgery, and who had not received prior treatment for their advanced disease, were either treated with Kimmtrak or the investigator’s choice of pembrolizumab (Keytruda), ipilimumab (Yervoy), or dacarbazine.
At a median follow-up of 14 months:
What are the potential side effects?
The most common side effects of Kimmtrak include skin reactions (dry skin, rash, itching, swelling), fever, tiredness, nausea, vomiting, chills, stomach pain, headache, low blood pressure, and abnormal liver blood tests. Some side effects, such as cytokine release syndrome, may be severe and life-threatening. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
Cytokine release syndrome (CRS)
CRS is caused by a widespread release of molecules called cytokines, which can cause inflammation and can affect the function of various organs. Cytokines may be released by the T cells to which Kimmtrak binds, or by other immune cells in the patient’s body. Signs and symptoms of CRS include fever, headache, nausea, low blood pressure, and weakness. A healthcare provider should be immediately notified if symptoms occur. CRS usually occurs at the beginning of the treatment, during the first three infusions.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Immunocore
Approval
FDA and EMA
Links to drug websites
- US: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761228s000lbl.pdf?utm_source=salesforce-marketing-cloud&utm_medium=email&utm_campaign=SPGA+-+FDA+Alert+-+01.26.2022&utm_term=View+full+prescribing+information+for+Kimmtrak
- Europe: https://www.ema.europa.eu/en/medicines/human/EPAR/kimmtrak
Other references
- Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. Nathan P, et al. The New England Journal of Medicine (2021)
Last updated on January 31, 2022