How is the drug name pronounced?
What cancer(s) does this drug treat?
Diffuse large B-cell lymphoma (DLBCL)
Monjuvi is approved for:
- Patients with diffuse large B-cell lymphoma who have previously received treatment, but the cancer either did not respond to treatment (refractory), or has since returned (relapsed), and for whom stem cell transplant is not an option. Monjuvi is given in combination with lenalidomide (Revlimid).
Limitations of Use
Age: The safety and efficacy of Monjuvi in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: The risks associated with Monjuvi during pregnancy are not known and cannot be ruled out. Monjuvi is not recommended use during pregnancy, and women are advised to use contraception during treatment and for at least 3 months after the last dose of Monjuvi. Monjuvi is given in combination with lenalidomide (Revlimid), which can cause harm to a fetus, including severe, life-threatening birth defects, and is not recommended for use during pregnancy. Women and men are advised to also refer to the lenalidomide prescribing information for contraception requirements. The risks associated with Monjuvi during breastfeeding are unknown, and cannot be ruled out. Due to the potential for adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for 3 months after the last dose of Monjuvi. Patients should refer to the lenalidomide prescribing information for additional recommendations.
What type of immunotherapy is this?
- Cell-killing antibody
How does this drug work?
Target:
- CD19 on B cells
Monjuvi is an antibody that was made in a laboratory and designed to attach to a protein molecule called CD19. CD19 is present on the surface of diffuse large B-cell lymphoma (DLBCL) cells. It is also found on the surface of normal human B cells, which means that in addition to attacking cancer cells, Monjuvi can also attack healthy B cells.
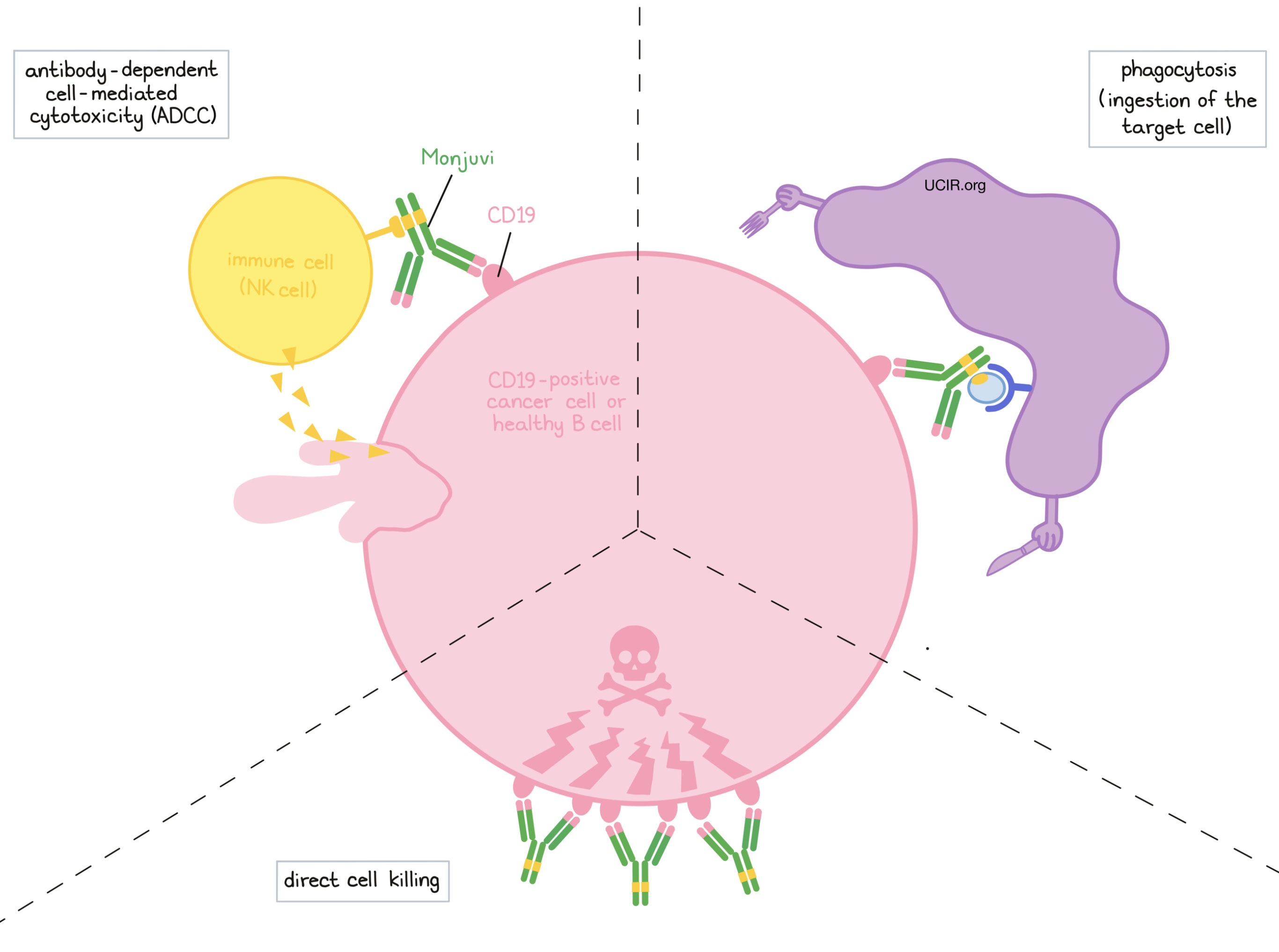
Monjuvi and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. For Monjuvi, the tips of the upper arms bind to CD19. The stem of Monjuvi’s “Y” shape has binding sites for immune cells or other parts of the immune system.
Monjuvi works to kill cancer cells in at least three ways:
Direct cell killing
By binding to CD19 molecules on the surface of diffuse large B-cell lymphoma (DLBCL) cells or normal B cells, Monjuvi can directly cause the cells to die.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to CD19 on the surface of diffuse large B-cell lymphoma cells or normal B cells, the stem of Monjuvi can also attract and bind immune cells (like NK cells). This allows Monjuvi to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell Monjuvi is bound to.
Antibody-dependent cell-mediated phagocytosis (ADCP)
When Monjuvi is bound to diffuse large B-cell lymphoma (DLBCL) cells or normal B cells, it can also attract immune cells called phagocytes, which have the ability to ingest (“eat”) cells that have been coated with Monjuvi.
The combined effect of these mechanisms results in the elimination of cancerous and normal B cells from the body. A new population of healthy B cells can then develop from blood-forming stem cells that reside in the bone marrow.
Lenalidomide has been shown to increase the activity of Monjuvi.
How is this drug given to the patient?
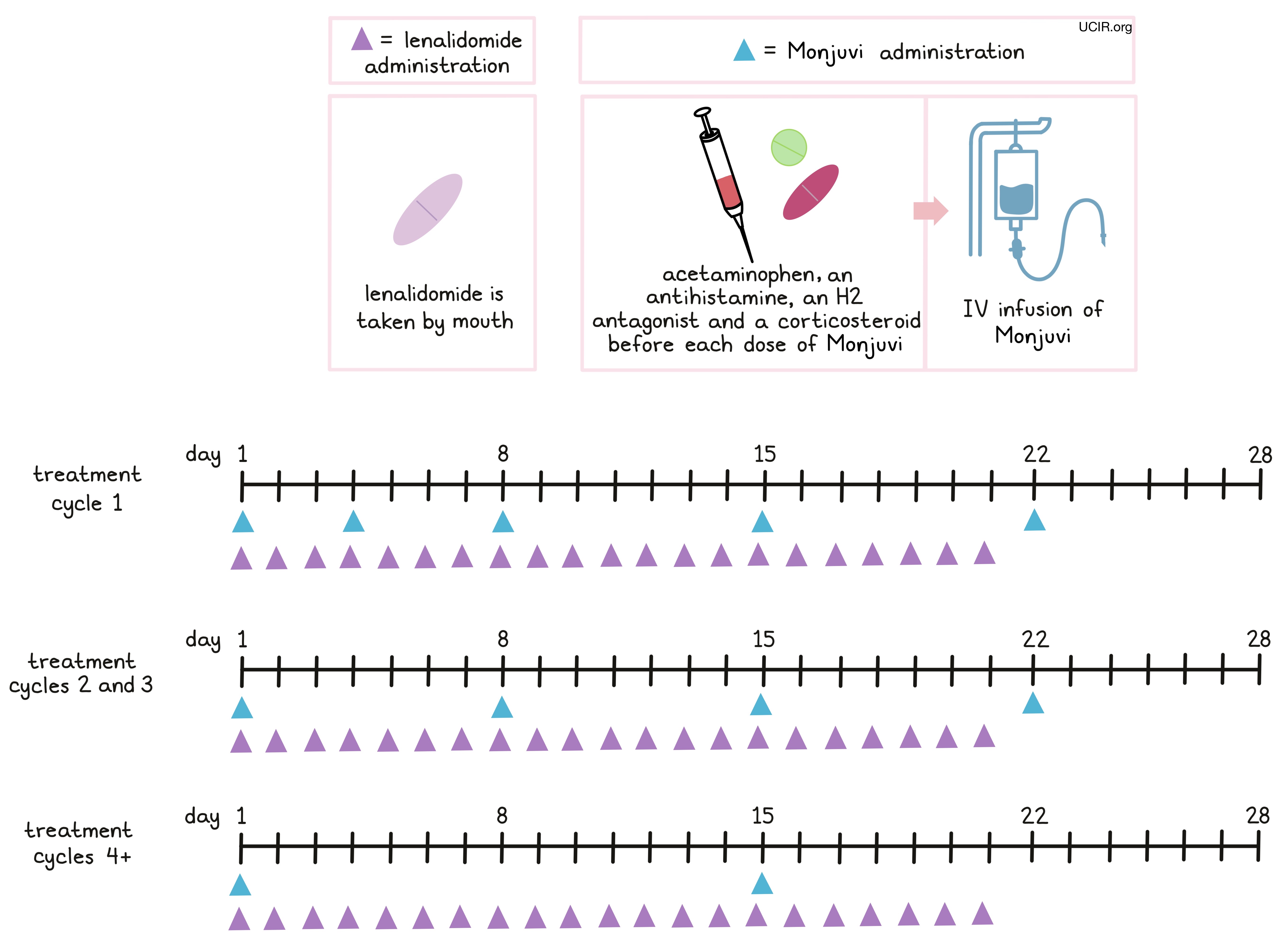
Monjuvi is administered through a tube in the vein (intravenous infusion, or i.v.). Treatment with Monjuvi is divided into 28-day treatment cycles.
- In the first treatment cycle, Monjuvi is administered on days 1, 4, 8, 15, and 22.
- In the second and third treatment cycles, Monjuvi is administered on days 1, 8, 15, and 22 (weekly).
- For treatment cycles four and up, Monjuvi is administered on days 1 and 15 (every other week).
Monjuvi is given in combination with lenalidomide (Revlimid) on days 1 through 21 of each 28-day treatment cycle for a maximum of 12 treatment cycles, followed by Monjuvi alone until the patient’s disease begins progressing or until the patient can no longer tolerate treatment. The first infusion of Monjuvi may take up to 2.5 hours to administer, but subsequent infusions typically last 1.5 to 2 hours.
Two hours to 30 minutes before the administration of Monjuvi patients may receive several medications to to help reduce the chance of and the severity of an reaction to the infusion:
- Acetaminophen,
- An antihistamine,
- An H2 antagonist, and/or
- Corticosteroids
If symptoms of an infusion-related reaction occur, the infusion may be slowed, interrupted, or permanently stopped, depending on the severity of the reaction. In order to ensure an immediate response to any infusion-related reactions, Monjuvi is administered in a hospital under the supervision of a healthcare professional.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Diffuse large B-cell lymphoma (Previously treated)
In a clinical trial, 71 patients with diffuse large B-cell lymphoma who had previously received treatment, but the cancer either did not respond (refractory), or had since returned (relapsed), and for whom stem cell transplant was not an option, were treated with Monjuvi in combination with lenalidomide.
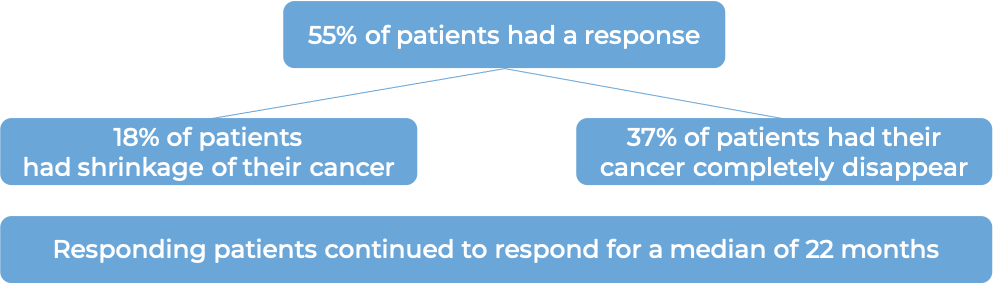
(For the definition of “median”, click HERE.)
What are the potential side effects?
Monjuvi targets the CD19 molecule, which, while present on diffuse large B-cell lymphoma (DLBCL) cells, is also present on normal B cells. As a result, Monjuvi can kill normal B cells, increasing the risk of serious infections. Other common side effects of Monjuvi include fever, fatigue, diarrhea, cough, decreased appetite, low white blood cell count, low red blood cell count, low platelet count, and swelling of lower legs or hands.
Some side effects, such as reactions related to the infusion, decreased bone marrow activity, and infections can become serious or life-threatening. Patients and caregivers receive careful instructions to monitor for signs and symptoms related to these conditions. These conditions are managed by the health care provider.
Infusion-Related Reactions
Infusion-related reactions are negative responses to receiving an infusion. Most infusion-related reactions typically occur within 24 hours of the first or second infusion. Symptoms include chills, fever, rash, flushing, high blood pressure, and shortness of breath. Palpitations (feeling as if your heart is fluttering or racing) and chest pain can also be symptoms of a reaction. A healthcare provider should be immediately notified if symptoms occur.
Decreased bone marrow activity (myelosuppression)
Monjuvi can suppress bone marrow activity and the formation of new blood cells, resulting in dangerously low blood cell counts. This may include low white blood cell, red blood cell, and/or platelet count. Symptoms of low blood cell counts include frequent infections, frequent or easy bruising, fatigue, shortness of breath, and dizziness. Blood tests are performed prior to each infusion and throughout treatment with Monjuvi in order to monitor blood cell counts. Patients may also receive a medication called granulocyte colony-stimulating factor (G-CSF), to activate the growth of certain blood cells if their white blood cell count becomes too low.
Infections
A common side effect of treatment with Monjuvi is infection. Respiratory tract infection, urinary tract infection, bronchitis, common cold, and pneumonia were the most commonly reported infections while taking Monjuvi.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. Treatment with Monjuvi may be interrupted and later resumed, or permanently stopped depending on the severity of the reaction. For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer
MorphoSys
Approval
FDA and EMA (European product name: Minjuvi)
Website
Last updated: March 30, 2022


