How is this drug name pronounced?
Siltuximab: sil-TUK-sih-mab
Sylvant: SIL-vant
What cancer(s) does this drug treat?
Sylvant is approved for:
Multicentric Castleman’s disease
- Patients with multicentric Castleman’s disease who are not infected with human immunodeficiency virus (HIV) or human herpesvirus-8 (HHV-8).
Limitations of use:
Age: The safety and efficacy of Sylvant in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Sylvant may cause harm to a fetus, and is not recommended for use during pregnancy. Pregnancy should be prevented for at least three months after the last dose of Sylvant. The risks associated with Sylvant during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Sylvant.
Infections: Sylvant should not be administered to patients with severe infections until the infection goes away. Sylvant may mask signs of an infection (e.g., by reducing fever) and patients should be carefully monitored by a physician during treatment with Sylvant in order to detect infections.
Vaccinations: Patients should not receive live virus vaccinations (e.g., chickenpox or measles, mumps, and rubella (MMR)) during treatment with Sylvant.
Interactions with other drugs: Dose adjustments may become necessary for patients treated with CYP450 substrates (e.g., warfarin, cyclosporin, theophylline) or CYP3A4 substrate drugs (e.g., oral contraceptives, lovastatin, atorvastatin) at the start or discontinuation of treatment with Sylvant.
What type of immunotherapy is this?
How does this drug work?
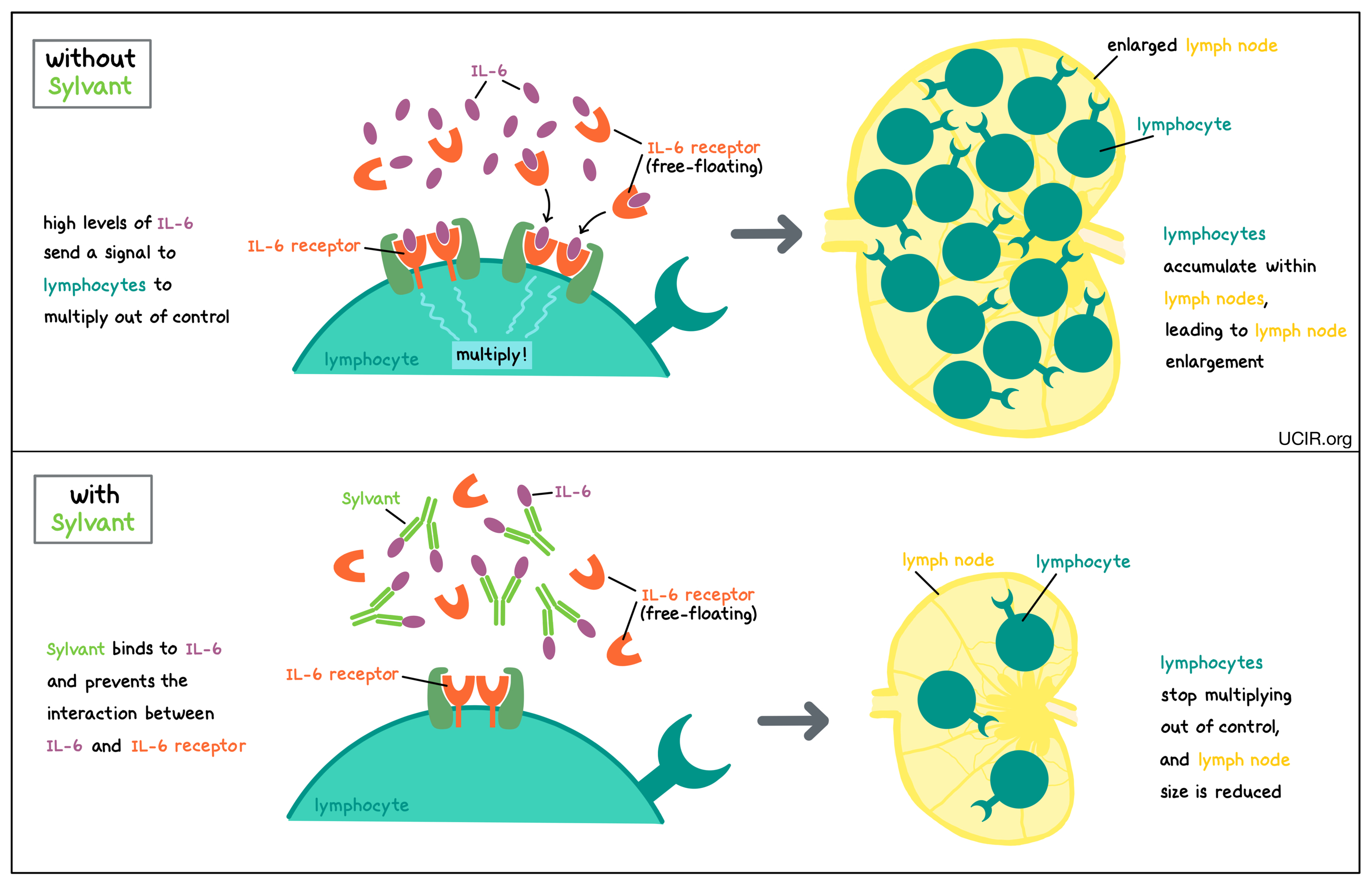
Sylvant is an antibody that attaches to a protein molecule called interleukin 6 (IL-6). IL-6 is produced by many different types of immune cells, including T cells, B cells, and monocytes, and when it is produced at normal levels, IL-6 plays a role in regulating the activity of the immune system. In multicentric Castleman’s disease, IL-6 is often present at higher than normal levels. Elevated levels of IL-6 cause lymphocytes (particularly B cells) to multiply too rapidly and accumulate within lymph nodes, leading to the enlargement of the lymph nodes and other symptoms of the disease.
Sylvant binds to IL-6 in such a way that prevents IL-6 from binding to the IL-6 receptor (which may be floating freely in body fluids or found on the surface of immune cells). This prevents lymphocytes from multiplying out of control, thus reducing the size of the lymph nodes and other symptoms of the disease.
How is this drug given to the patient?
Prior to the administration of each dose of Sylvant, patients are checked for blood cell counts and hemoglobin levels. This is done prior to every dose during the first year of treatment, and prior to every third dose after one year.
Sylvant is administered via a tube into a vein (intravenous infusion, or I.V.) over one hour every three weeks, and does not require a hospital stay.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Multicentric Castleman’s disease
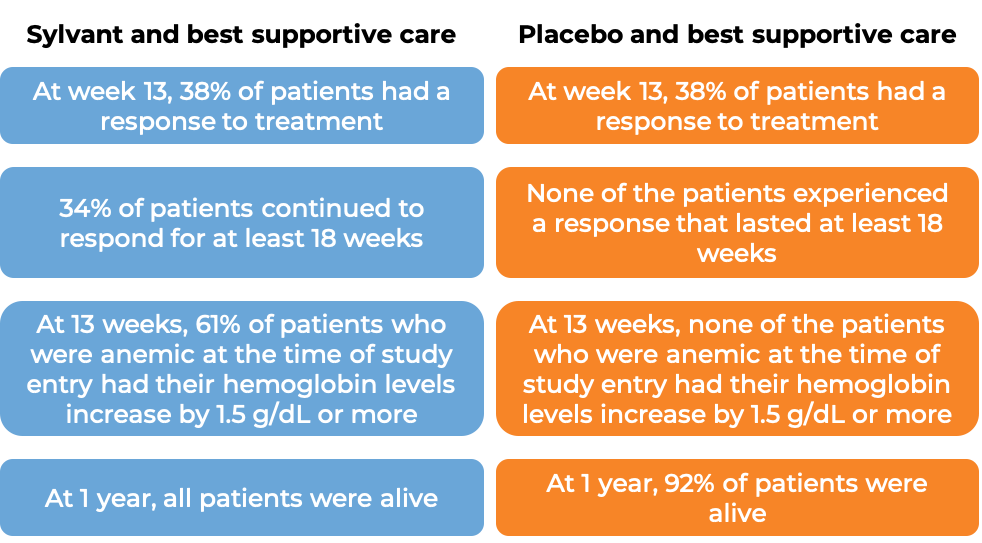
In a clinical trial, 79 patients with multicentric Castleman’s disease were treated with either:
- Sylvant and best supportive care, OR
- placebo and best supportive care
What are the side effects?
The most common side effects of Sylvant include rash, itching, upper respiratory tract infection, swelling of hands and feet, pain in limbs and joints, weight gain, diarrhea, and increased levels of uric acid in the blood. Other common side effects of Sylvant include urinary tract infection, kidney problems, low cell counts for infection-fighting neutrophils, low platelets, dizziness, headache, high blood pressure, nausea, abdominal pain, vomiting, acid reflux, heartburn, and canker sores.
Sylvant can cause side effects that can become serious or life-threatening. Some of the serious side effects related to Sylvant include severe infections, severe allergic reactions, reactions related to the infusion, and development of a hole in the stomach or intestine. Sylvant should be discontinued for patients who experience severe reactions related to the infusion or severe allergic reactions to the treatment.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Janssen Biotech in the US, EUSA Pharma in Europe
Approval
FDA and EMA
Links to drug websites
Last updated on January 15, 2021
How is this drug name pronounced?
Siltuximab: sil-TUK-sih-mab
Sylvant: SIL-vant
What cancer(s) does this drug treat?
Sylvant is approved for:
Multicentric Castleman’s disease
- Patients with multicentric Castleman’s disease who are not infected with human immunodeficiency virus (HIV) or human herpesvirus-8 (HHV-8).
Limitations of use:
Age: The safety and efficacy of Sylvant in patients under 17 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Sylvant may cause harm to a fetus, and is not recommended for use during pregnancy. Pregnancy should be prevented for at least three months after the last dose of Sylvant. The risks associated with Sylvant during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Sylvant.
Infections: Sylvant should not be administered to patients with severe infections until the infection goes away. Sylvant may mask signs of an infection (e.g., by reducing fever) and patients should be carefully monitored by a physician during treatment with Sylvant in order to detect infections.
Vaccinations: Patients should not receive live virus vaccinations (e.g., chickenpox or measles, mumps, and rubella (MMR)) within 4 weeks before or during treatment with Sylvant.
Interactions with other drugs: Dose adjustments may become necessary for patients treated with CYP450 substrates (e.g., warfarin, cyclosporin, theophylline) or CYP3A4 substrate drugs (e.g., oral contraceptives, lovastatin, atorvastatin) at the start or discontinuation of treatment with Sylvant.
What type of immunotherapy is this?
How does this drug work?
Sylvant is an antibody that attaches to a protein molecule called interleukin 6 (IL-6). IL-6 is produced by many different types of immune cells, including T cells, B cells, and monocytes, and when it is produced at normal levels, IL-6 plays a role in regulating the activity of the immune system. In multicentric Castleman’s disease, IL-6 is often present at higher than normal levels. Elevated levels of IL-6 cause lymphocytes (particularly B cells) to multiply too rapidly and accumulate within lymph nodes, leading to the enlargement of the lymph nodes and other symptoms of the disease.
Sylvant binds to IL-6 in such a way that prevents IL-6 from binding to the IL-6 receptor (which may be floating freely in body fluids or is found on the surface of the immune cells). This prevents lymphocytes from multiplying out of control, thus reducing the size of the lymph nodes and other symptoms of the disease.

How is this drug given to the patient?
Prior to the administration of each dose of Sylvant, patients are checked for blood cell counts and hemoglobin levels. This is done before every dose for the first year of treatment, and before every third dose after one year.
Sylvant is administered via a tube into a vein (intravenous infusion, or i.v.) over one hour every three weeks, and does not require a hospital stay.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Multicentric Castleman’s disease
In a clinical trial, 79 patients with multicentric Castleman’s disease were treated with either:
- Sylvant and best supportive care, OR
- placebo and best supportive care

In another clinical trial, 60 patients with multicentric Castleman’s disease were treated with Sylvant for a median of 5.5 years. At a median follow-up of 6 years, all patients were alive, and disease remained under control in 58 of 60 patients.
What are the side effects?
The most common side effects of Sylvant include rash, itching, upper respiratory tract infection, swelling of hands and feet, pain in limbs and joints, weight gain, diarrhea, and increased levels of uric acid in the blood. Other common side effects of Sylvant include urinary tract infection, kidney problems, low cell counts for infection-fighting neutrophils, low platelets, dizziness, headache, high blood pressure, nausea, abdominal pain, vomiting, acid reflux, heartburn, and canker sores.
Sylvant can cause side effects that can become serious or life-threatening. Some of the serious side effects related to Sylvant include severe infections, severe allergic reactions, and reactions related to the infusion. Sylvant should be discontinued for patients who experience severe reactions related to the infusion or severe allergic reactions to the treatment.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Janssen Biotech in the US, Recordati in Europe
Approval
FDA and EMA
Links to drug websites
Last updated on March 14, 2024




