Approval in the US
The US product name for Gazyvaro is Gazyva.
How is the drug name pronounced?
Obinutuzumab: oh-bi-noo-tooz-oo-mab
Gazyvaro: guh-ZY-var-OH
The US product name for Gazyvaro is Gazyva.
What cancer(s) does this drug treat?
Chronic lymphocytic leukemia (CLL)
Gazyvaro is approved for:
- Patients with previously untreated chronic lymphocytic leukemia (CLL) who have additional conditions that make them unfit for treatment with the chemotherapy drug fludarabine. In such cases, Gazyvaro is used in combination with the chemotherapy drug chlorambucil.
Follicular lymphoma (FL)
Gazyvaro is approved for:
- Patients with previously untreated advanced follicular lymphoma. In such cases, Gazyvaro is given in combination with chemotherapy, followed by maintenance therapy with Gazyvaro alone to prevent the cancer from getting worse or coming back.
- Patients with follicular lymphoma who were previously treated with rituximab (e.g., MabThera, Truxima) but their cancer either did not respond to treatment (refractory) or worsened (relapsed) during or within six months following treatment . In such cases, Gazyvaro is given in combination with bendamustine, followed by maintenance therapy with Gazyvaro alone to prevent the cancer from getting worse or coming back.
Limitations of Use
Age: The safety and efficacy of Gazyvaro has not been established in patients under 18 years of age.
Vaccinations: Live virus vaccinations (e.g., chickenpox or measles, mumps, and rubella (MMR)) prior to or during Gazyvaro treatment are not recommended.
Pregnancy/breastfeeding: Gazyvaro may cause harm to a fetus and is not recommended for use during pregnancy. Women who are able to become pregnant should use effective birth control while receiving Gazyvaro, and for 18 months after the last dose of Gazyvaro. Due to the potential for serious adverse reactions in a breastfed child, women are also advised not to breastfeed during treatment and for at least 18 months after the last dose of Gazyvaro.
What type of immunotherapy is this?
- Cell-killing antibody
How does this drug work?
Target:
- CD20 molecule on B cells
Gazyvaro is an antibody that was made in the laboratory. Gazyvaro and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. Gazyvaro binds to a molecule called CD20 on the surface of a cancerous B cell. CD20 is commonly found on the surface of cancer cells in patients with Non-Hodgkin lymphoma (including Follicular lymphoma) and Chronic Lymphocytic Leukemia. CD20 can also be found on the surface of all normal human B cells, which means that in addition to attacking cancer cells, Gazyvaro can also attack healthy B cells.
The stem of Gazyvaro’s “Y” shape has binding sites for immune cells or other parts of the immune system.
At least four different mechanisms are thought to be responsible for the elimination of CD20-positive B cells by Gazyvaro. Gazyvaro is helped by the immune system to kill cancer cells and may also work alone.
Complement-dependent cytotoxicity (CDC)
When bound to CD20 on the surface of cancerous or normal B cells, the “stem” of Gazyvaro can attract and bind molecules of the immune system called complement that freely flow in the blood or tissues. Activation of the complement system causes the formation of the “membrane attack complex”, which can puncture and destroy the cell that Gazyvaro is bound to.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to CD20 on the surface of cancerous or normal B cells, the “stem” of Gazyvaro can also attract and bind immune cells (like NK cells). This allows Gazyvaro to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell Gazyvaro is bound to. Because of its reduced fucose content (a sugar that inhibits binding to certain immune cells, such as NK cells), Gazyvaro more often induces ADCC than comparable anti-CD20 treatments, such as rituximab (e.g., MabThera, Truxima).
Phagocytosis
When Gazyvaro is bound to cancerous or normal B cells, it can also attract immune cells called phagocytes that have the ability to ingest (“eat”) cells that have been coated with Gazyvaro or parts of the complement system.
Direct cell killing
By binding to CD20 molecules on the surface of cancerous or normal B cells and bringing the CD20 molecules close together in “clusters”, Gazyvaro can directly cause the cells to die. Gazyvaro has been shown to be more effective at directly killing cells through this mechanism than comparable anti-CD20 treatments, such as rituximab (e.g., MabThera, Truxima).
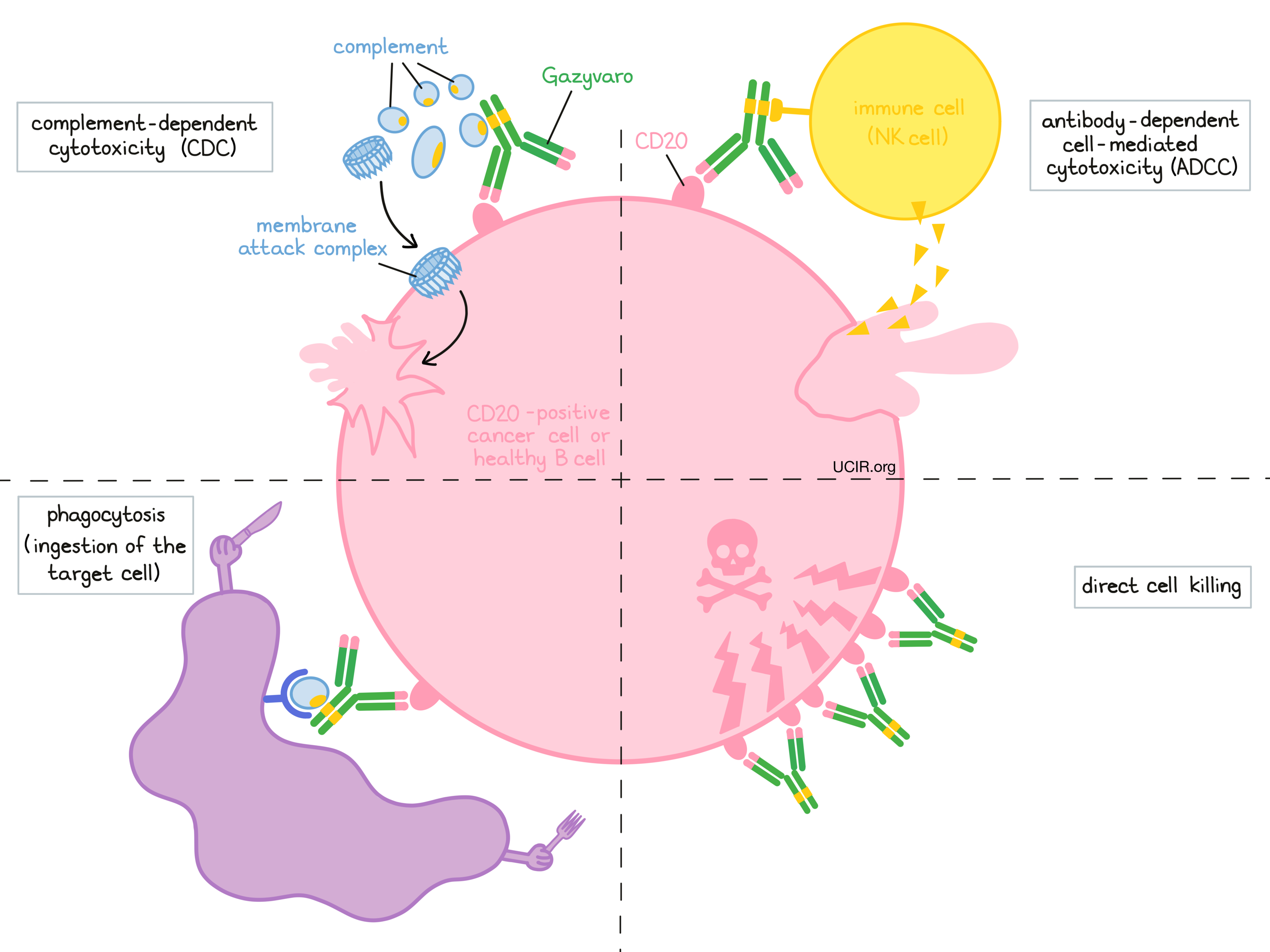
The combined effect of these mechanisms results in the elimination of cancerous and normal B cells from the body. A new population of healthy B cells can then develop from blood-forming ‘stem’ cells that reside in the bone marrow.
How is this drug given to the patient?
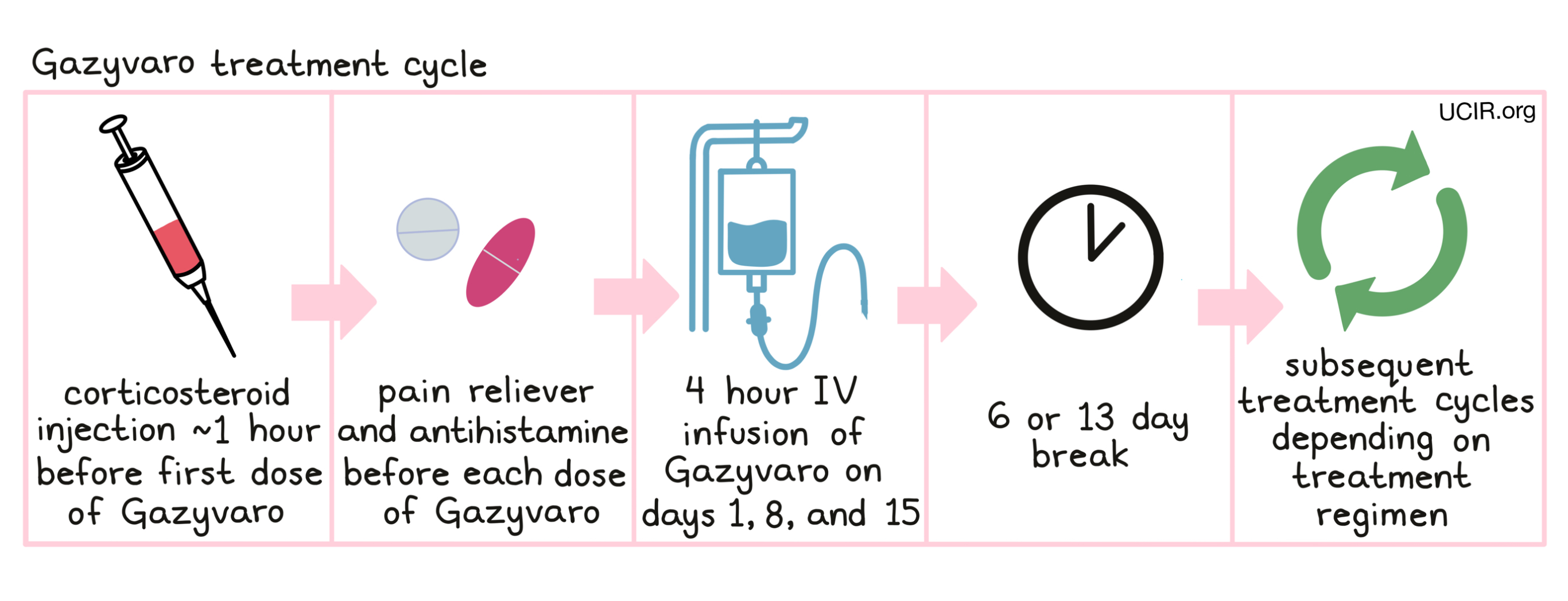
Gazyvaro is administered via a tube in the vein (intravenous infusion, or i.v.) over 4 or more hours once a week for 3 weeks on days 1*, 8 and 15, followed by 6 or 13 days with no treatment. Each 21- or 28-day (3 or 4 week) period is one “treatment cycle”. The number of treatment cycles, the length of each cycle, and the duration of treatment depends on the patient’s cancer and the treatment they are receiving.
*The first dose of Gazyvaro for chronic lymphocytic leukemia patients is divided over two days (days 1 and 2).
Administration of Gazyvaro does not require a hospital stay. Gazyvaro infusions will be initiated at a low rate, which may be increased every 30 minutes. Prior to the first infusion and, if indeed, subsequent infusions, the patient will receive
- a corticosteroid such as dexamethasone or methylprednisolone intravenously,
- a pain reliever such as paracetamol by mouth, and
- an antihistamine such as diphenhydramine by mouth or intravenously
in order to reduce the risk of infusion-related side effects.
If symptoms of an infusion-related reaction occur at any point during the first infusion or any subsequent infusions, the infusion may be interrupted or slowed, depending on the severity of the reaction. It is important that patients discuss with their doctor if they notice any changes following a Gazyvaro infusion, as infusion-related reactions are very serious.
Before starting Gazyvaro therapy, patients will need to get blood tests done to check if the patient is a carrier of the hepatitis B virus. Gazyvaro can cause the virus to become active again, and patients who test positive for the hepatitis B virus will need to be monitored more closely during treatment and for several months afterward. Blood tests are also used to obtain complete blood counts on a regular basis during treatment and after ending treatment. This is because reductions in the number of blood cells are particularly common in patients being treated with Gazyvaro.
Patients with a high tumor burden, high numbers of lymphocytes in the blood, or underlying problems with kidney function should receive additional treatment (“prophylaxis”) prior to receiving Gazyvaro to prevent tumor lysis syndrome, a serious side effect caused by the rapid breakdown of cancerous cells. Prophylaxis consists of adequate hydration and administration of drugs that help to prevent buildup of uric acid in the blood, such as allopurinol or rasburicase, 12-24 hours prior to the first infusion. If the patient is still at risk for tumor lysis syndrome, they may also continue to receive prophylaxis prior to subsequent infusions.
Patients with severe and long-lasting low cell counts for infection-fighting neutrophils (neutropenia) may be treated with drugs that fight off bacteria, fungi, or viruses as a precaution to prevent infections.
What are the observed clinical results?
For:
Chronic lymphocytic leukemia
Follicular lymphoma (previously untreated)
Follicular lymphoma (previously treated with rituximab)
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Chronic Lymphocytic Leukemia
In a clinical study of 781 patients with previously untreated chronic lymphocytic leukemia (CLL), patients were treated with either Gazyvaro in combination with chlorambucil, a rituximab product (e.g., MabThera, Truxima) in combination with chlorambucil, or with chlorambucil alone.
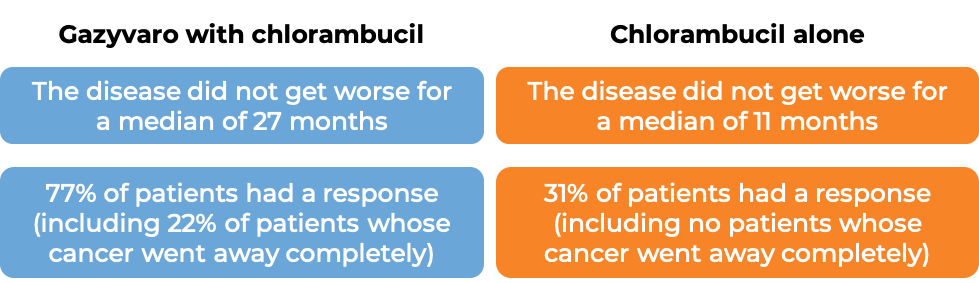
The first stage of the study compared Gazyvaro with chlorambucil to chlorambucil alone. At a median follow-up of 23 months:
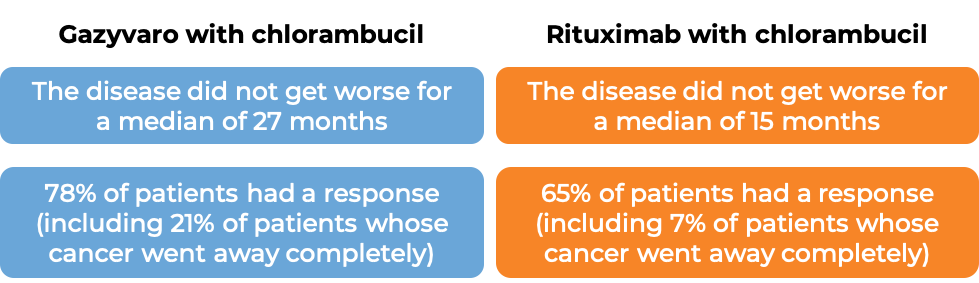
The second stage of the study compared Gazyvaro with chlorambucil to rituximab with chlorambucil. (The Gazyvaro with chlorambucil group included all patients from the first stage of the study plus additional recruited patients.) At a median follow-up of 19 months:
Follicular lymphoma (previously untreated)
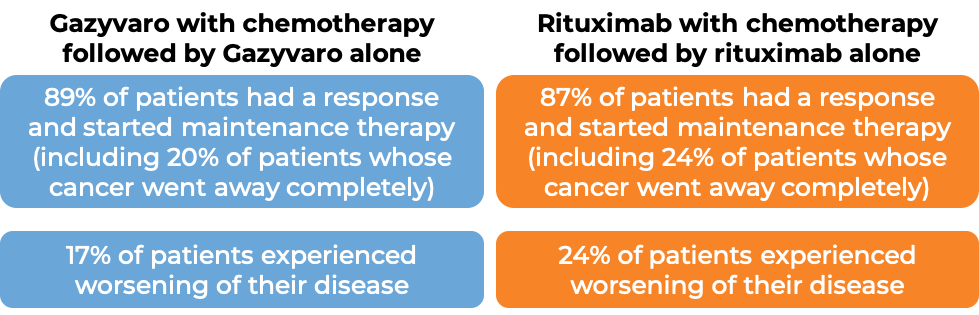
In a clinical study of 1,202 patients with previously untreated, advanced follicular lymphoma, patients were treated with either Gazyvaro in combination with chemotherapy, or a rituximab product (e.g., MabThera, Truxima) in combination with chemotherapy (bendamustine; cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP]; or cyclophosphamide, vincristine, and prednisone [CVP]). Following initial treatment, patients whose cancer extent decreased or whose cancer went away completely were given maintenance therapy (Gazyvaro alone for patients who responded to Gazyvaro combination therapy, or rituximab alone for patients who responded to rituximab combination therapy). At a median follow-up of 34 to 35 months:
Follicular lymphoma (previously treated with rituximab)
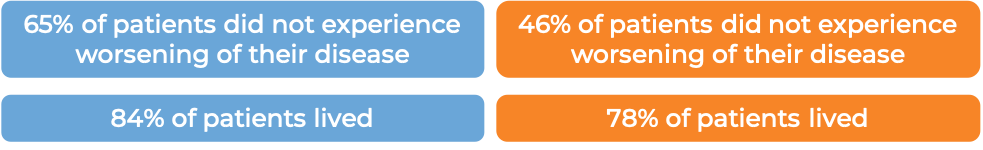
In a clinical study of 321 patients with follicular lymphoma who were previously treated with rituximab (e.g., MabThera, Truxima), but their cancer either did not respond to treatment or worsened within 6 months following treatment, patients were treated with either Gazyvaro in combination with bendamustine, followed by maintenance therapy with Gazyvaro alone to prevent the cancer from getting worse or coming back, or with bendamustine alone.
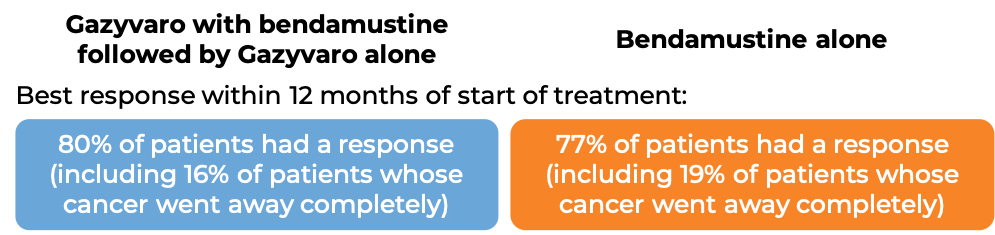
At a median follow-up of 20-22 months:
What are the potential side effects?
Gazyvaro targets the CD20 molecule which, while present on leukemic B cells, is also present on normal B cells. As a result, Gazyvaro can kill normal B cells, increasing the risk of infections, including urinary and respiratory infections. Other common side effects of Gazyvaro include low blood cell counts (in particular, low counts for infection-fighting neutrophils or platelets), skin rashes, insomnia, headache, back pain, weakness, nausea, fever, cough, and diarrhea.
Some side effects, such as reactions related to the infusion, severe infections, infections of the brain, hepatitis B virus reactivation, tumor lysis syndrome, heart problems, and kidney problems, may be severe or life-threatening. Patients and caregivers receive careful instructions to monitor for signs and symptoms of these complications, and a healthcare provider should be immediately notified if symptoms occur. These conditions are managed by the health care provider.
Infusion-related reactions
Reactions to the Gazyvaro infusion are common. Some of these reactions are serious and life-threatening. Symptoms include hives, rashes, swelling of lips, tongue, or face, sudden cough, shortness of breath, difficulty breathing, dizziness or feeling faint, heart racing or fluttering, and chest pain.
Hypersensitivity reactions
Hypersensitivity reactions to Gazyvaro are allergic reactions and include shortness of breath, difficulty breathing, hives, low blood pressure, and fast heart rate. In the event of hypersensitivity reactions to Gazyvaro, treatment will be permanently discontinued.
Serious infections and hepatitis B virus reactivation
Gazyvaro can reduce the immune system’s ability to fight off bacterial, fungal, and viral infections, can increase a patient’s risk of getting an infection, and can cause the reactivation of prior viral infections. These infections can be serious, and may be fatal. Symptoms of infection include: fever; cold symptoms, such as a persistently runny nose or sore throat; flu symptoms, such as coughing, tiredness, and achiness; earache or headache; painful urination; cold sores; and cuts or scrapes that are red, warm, swollen, or painful. If the patient is a carrier of the hepatitis B virus, Gazyvaro can cause the virus to become active. Hepatitis B can cause serious liver problems, including liver failure and death.
Tumor Lysis Syndrome (TLS)
TLS is caused by the rapid breakdown of cancer cells. TLS can cause kidney failure and abnormal heart rhythms. Symptoms of TLS include nausea, vomiting, diarrhea, and lethargy.
Progressive Multifocal Leukoencephalopathy (PML)
PML is a rare infection of nerve cells by a virus that can be contracted by individuals with weakened immune systems. Symptoms of PML include confusion, dizziness or loss of balance, difficulty walking or talking, weakness, and vision problems.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Roche
Approval
FDA (US product name is Gazyva) EMA (European product name is Gazyvaro)
Website
Last updated on August 11, 2025


