How is the drug name pronounced?
Nivolumab and relatlimab-rmbw: nih-VOL-yoo-mab and reh-LAT-lih-mab
Opdualag: Op-DOO-ah-lag
What cancer(s) does this drug treat?
Melanoma
Opdualag is approved for:
- adult and pediatric patients who are 12 years or older with melanoma that cannot be removed by surgery, or that has spread to other parts of the body.
Limitations of use:
Age: The safety and efficacy of Opdualag have not been established in patients under 12 years of age or less than 40 kg in body weight.
Pregnancy/Breastfeeding: Opdualag may cause harm to the fetus and is not recommended for use during pregnancy. Women are advised to use effective contraception during treatment with Opdualag and for at least 5 months after the last dose of Opdualag. The risks associated with Opdualag in a breastfed child are not known and cannot be ruled out. Due to the potential for serious reactions in the breastfed child, patients are advised not to breastfeed during treatment with Opdualag and for 5 months after the last dose of Opdualag.
Complications of stem cell transplant: Serious and life-threatening complications that can lead to death can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Opdualag. The benefit of treatment with Opdualag versus the possible risk of transplant-related complications (especially in patients with a history of graft-versus-host disease) should be carefully considered.
What type of immunotherapy is this?
How does this drug work?
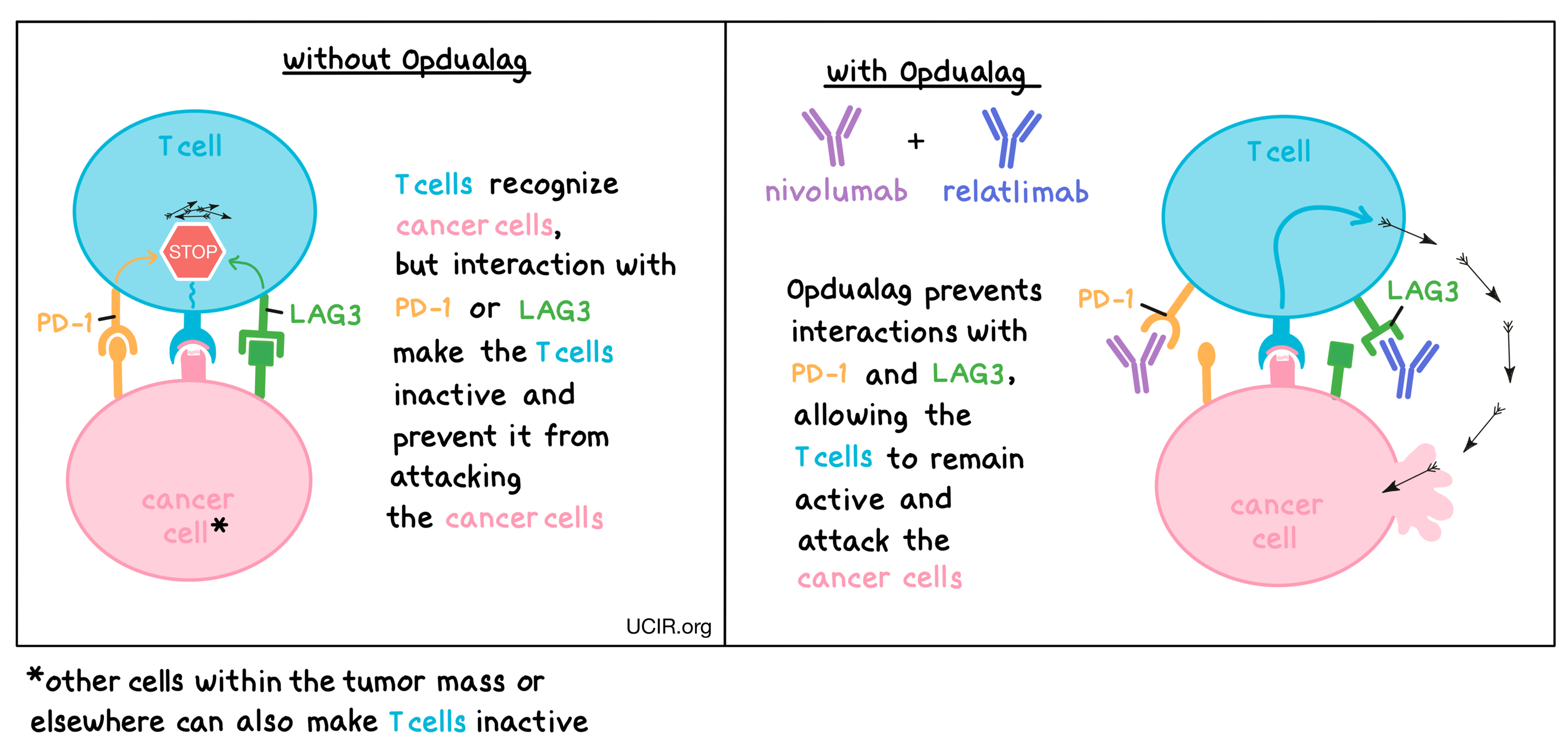
Targets:
Opdualag is a checkpoint blockade therapy consisting of two types of therapeutic antibodies – nivolumab and relatlimab. Nivolumab attaches to a molecule called PD-1, while relatlimab binds to a molecule called LAG3, both of which are present on the surfaces of T cells – the primary immune cells involved in killing cancer cells. In healthy tissues, PD-1 and LAG3 act as checkpoints or brakes that keep T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from killing the cancer cells. This can happen when cancer cells or other cells within the tumor mass display molecules on their surface that interact with PD-1 or LAG3, as these interactions cause the T cells to become inactive, preventing them from attacking the cancer cells. Nivolumab and relatlimab bind to PD-1 and LAG3, respectively, preventing such interactions and allowing T cells to remain active and attack the cancer cells. Combining nivolumab and relatlimab into Opdualag results in an even stronger effect on the anti-cancer T cells than use of either antibody alone.
How is this drug given to the patient?
Opdualag is administered via a tube in the vein (intravenous infusion, or i.v.) once every 4 weeks. Each infusion of Opdualag lasts for about 30 minutes and does not require a hospital stay.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
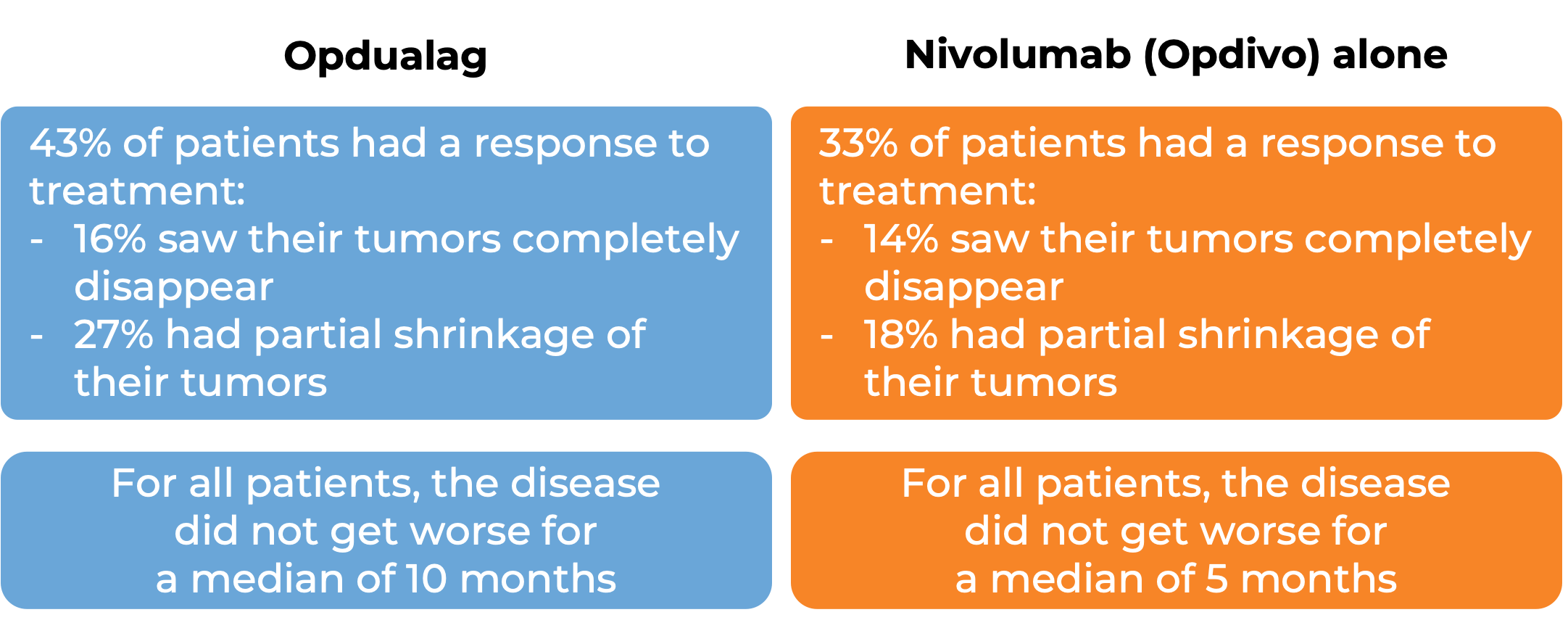
Advanced melanoma
In a clinical trial, 714 patients with advanced melanoma that had spread to other parts of the body (metastatic) or could not be removed by surgery (unresectable), and who had not received prior treatment for their advanced disease were treated with either Opdualag or nivolumab (Opidvo). At a median follow-up of 19 months:
What are the potential side effects?
The most common side effects of Opdualag include muscle and joint pain, fatigue, low white blood cell counts, abnormal liver function test results, rash, itchiness, diarrhea, and abnormal blood test results.
Opdualag can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Opdualag can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Opdualag include inflammation of the lungs, liver, brain, kidneys (which can lead to kidney failure), colon (which can result in tears or holes in the intestine), or heart. Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Opdualag may cause Type 1 diabetes. Skin rash (which could become severe and life-threatening), and reactions related to the infusion may also occur. Symptoms of an infusion-related reaction may include chills or shaking, itching or rash, flushing, shortness of breath, dizziness, lightheadedness, fever, and back or neck pain.
Patients who experience side effects should report them to their healthcare provider who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Bristol-Myers Squibb
Approval
FDA and EMA
Links to drug websites
Other references:
Last updated: May 31, 2022
How is the drug name pronounced?
Nivolumab and relatlimab-rmbw: nih-VOL-yoo-mab and reh-LAT-lih-mab
Opdualag: Op-DOO-ah-lag
What cancer(s) does this drug treat?
Melanoma
Opdualag is approved for:
- Adult and pediatric patients who are 12 years or older with melanoma that cannot be removed by surgery, or has spread to other parts of the body, and for whom lab tests reveal low levels of the molecule PD-L1 in the tumor.
Limitations of use:
Age: The safety and efficacy of Opdualag have not been established in patients under 12 years of age.
Pregnancy/Breastfeeding: Opdualag may cause harm to the fetus and is not recommended for use during pregnancy. Women are advised to use effective contraception during treatment with Opdualag and for at least 5 months after the last dose of Opdualag. The risks associated with Opdualag in a breastfed child are not known and cannot be ruled out. Due to the potential for serious reactions in the breastfed child, patients are advised not to breastfeed during treatment with Opdualag and for 5 months after the last dose of Opdualag.
Complications of stem cell transplant: Serious and life-threatening complications that can lead to death can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Opdualag. The benefit of treatment with Opdualag versus the possible risk of transplant-related complications (especially in patients with a history of graft-versus-host disease) should be carefully considered.
Solid organ transplant rejection: Treatment with Opdualag can increase the risk of rejection in patients who have received an organ transplant.
Interactions with other drugs: Corticosteroids or other drugs that can suppress an immune response should not be administered shortly before treatment with Opdualag, as they may limit the activity of Opdualag. However, once Opdualag treatment has started, corticosteroids may be used to reduce side effects.
What type of immunotherapy is this?
How does this drug work?
Targets:
Opdualag is a checkpoint blockade therapy consisting of two types of therapeutic antibodies – nivolumab and relatlimab. Nivolumab attaches to a molecule called PD-1, while relatlimab binds to a molecule called LAG3, both of which are present on the surfaces of T cells – the primary immune cells involved in killing cancer cells. In healthy tissues, PD-1 and LAG3 act as checkpoints or brakes that keep T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from killing the cancer cells. This can happen when cancer cells or other cells within the tumor mass display molecules on their surface that interact with PD-1 or LAG3, as these interactions cause the T cells to become inactive, preventing them from attacking the cancer cells. Nivolumab and relatlimab bind to PD-1 and LAG3, respectively, preventing such interactions and allowing T cells to remain active and attack the cancer cells. Combining nivolumab and relatlimab into Opdualag results in an even stronger effect on the anti-cancer T cells than use of either antibody alone.

How is this drug given to the patient?
Opdualag is administered via a tube in the vein (intravenous infusion, or i.v.) once every 4 weeks. Each infusion of Opdualag lasts for about 30 minutes and does not require a hospital stay.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
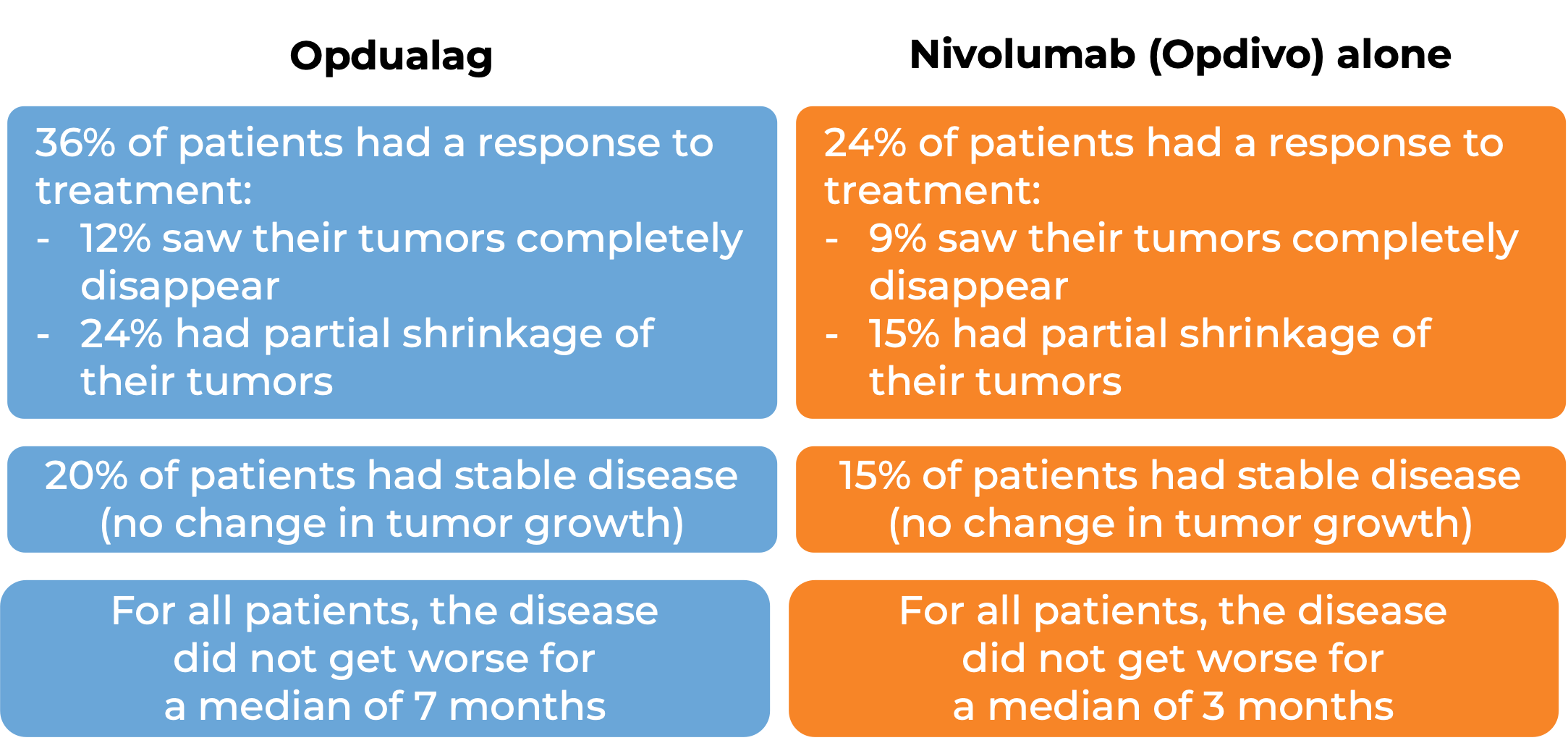
Advanced melanoma
In a clinical trial, 421 patients with advanced melanoma that had spread to other parts of the body (metastatic) or could not be removed by surgery (unresectable), for whom lab tests revealed low levels of the molecule PD-L1 in the tumor, and who had not received prior treatment for their advanced disease were treated with either Opdualag or nivolumab (Opidvo). At a median follow-up of 18 months:
What are the potential side effects?
The most common side effects of Opdualag include muscle and joint pain, fatigue, low white blood cell counts, abnormal liver function test results, rash, itchiness, diarrhea, and abnormal blood test results.
Opdualag can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Opdualag can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Opdualag include inflammation of the lungs, liver, brain, kidneys (which can lead to kidney failure), colon (which can result in tears or holes in the intestine), or heart. Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Opdualag may cause Type 1 diabetes. Skin rash (which could become severe and life-threatening), and reactions related to the infusion may also occur. Symptoms of an infusion-related reaction may include chills or shaking, itching or rash, flushing, shortness of breath, dizziness, lightheadedness, fever, and back or neck pain.
Patients who experience side effects should report them to their healthcare provider who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Bristol-Myers Squibb
Approval
FDA and EMA
Links to drug websites
Other references:
Last updated: November 26, 2022





