How is the drug name pronounced?
Danyelza: dan-YEL-zah
Naxitamab: nak-SIH-tuh-mab
What cancer(s) does this drug treat?
Neuroblastoma (in the bone or bone marrow)
Danyelza is approved for:
- Patients 1 year of age or older with neuroblastoma in the bone or bone marrow who have responded to a prior treatment, but whose cancer has since returned (relapsed) or stopped responding to the prior treatment (refractory). Danyelza is used in combination with the cytokine drug granulocyte-macrophage colony-stimulating factor (GM-CSF).
Limitations of Use
Age: The safety and efficacy of Danyelza in patients under 1 year of age have not been established.
Pregnancy/Breastfeeding: The risks associated with Danyelza during pregnancy are not known and cannot be ruled out. Due to the potential for harm to the fetus, Danyelza is not recommended for use during pregnancy. Women are advised to use contraception during treatment and for at least 2 months after the last dose of Danyelza. The risks associated with Danyelza during breastfeeding are not known and cannot be ruled out. Due to the potential for negative reactions in the breastfed child, women are advised not to breastfeed during treatment, and for 2 months after the last dose of Danyelza.
What type of immunotherapy is this?
- Cell-killing antibody
How does this drug work?
Target:
- GD2
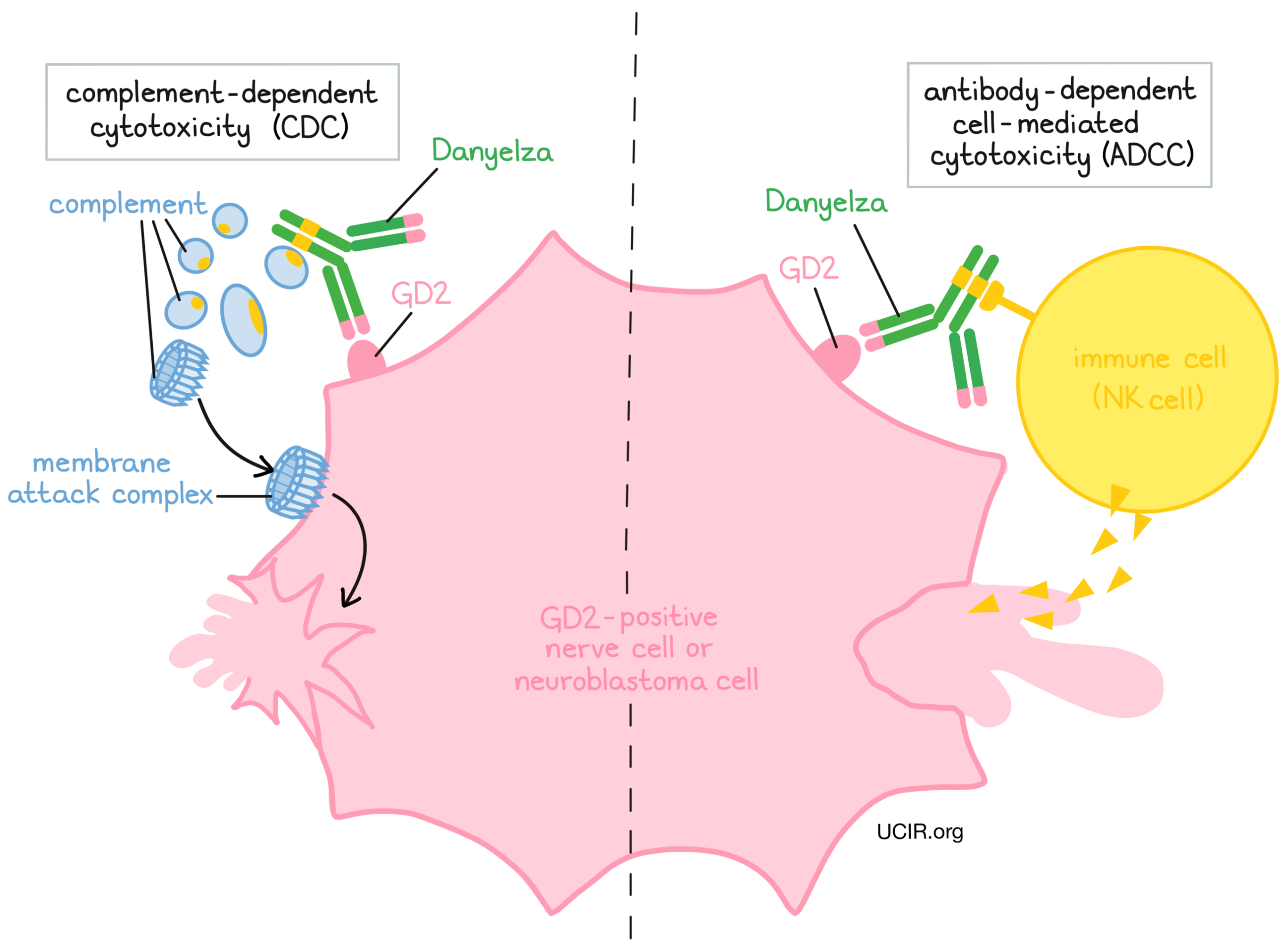
Danyelza is an antibody that was made in a laboratory and designed to attach to a protein molecule called GD2. GD2 is present on the surface of nerve cells, but is present in much higher quantities on the surface of neuroblastoma cells. Higher-than-normal amounts of GD2 on neuroblastoma cells make these cells the main targets of Danyelza.
Danyelza and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. For Danyelza, the tips of the upper arms bind to GD2. The stem of Danyelza’s “Y” shape has binding sites for immune cells or other parts of the immune system.
Danyelza works to kill cancer cells in at least two ways:
Complement-dependent cytotoxicity (CDC)
When bound to GD2 on the surface of neuroblastoma cells, the stem of Danyelza can attract and bind molecules of the immune system called “complement” that freely flow in the blood or tissues. Activation of the complement system causes the formation of the “membrane attack complex”, which can puncture and destroy the cell that Danyelza is bound to.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to GD2 on the surface of neuroblastoma cells, the stem of Danyelza can also attract and bind immune cells (like NK cells). This allows Danyelza to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell Danyelza is bound to.
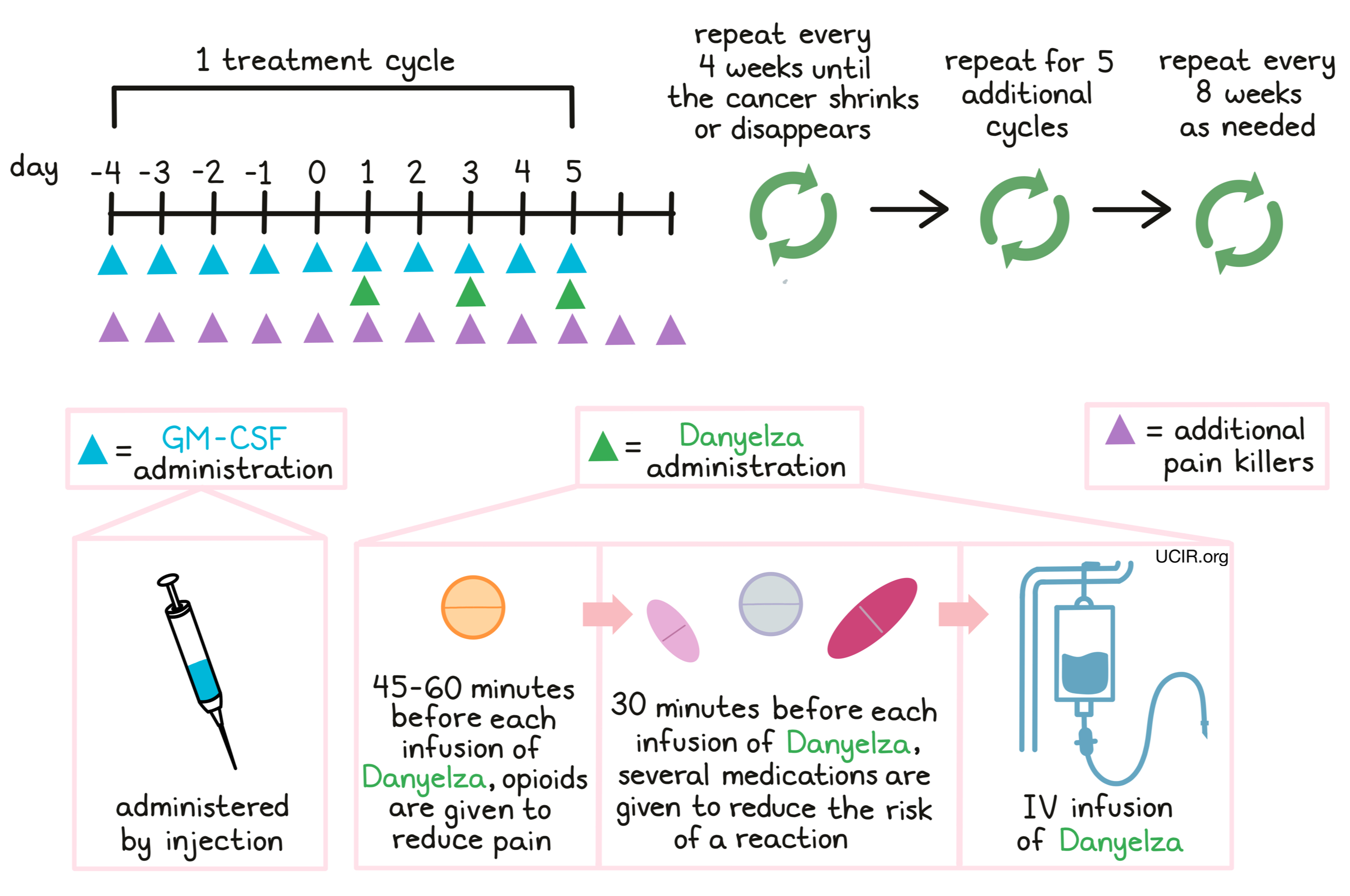
How is this drug given to the patient?
Danyelza is administered through a tube in the vein (intravenous infusion, or i.v.) on days 1, 3, and 5 of each 10-day treatment cycle. Patients receiving treatment with Danyelza also receive a injection of granulocyte-macrophage colony-stimulating factor (GM-CSF) under the skin (subcutaneous) once daily, beginning five days prior to the first administration of Danyelza within a treatment cycle, and continuing for the duration of the cycle. During each treatment cycle and two more days thereafter, patients receive medication daily, such as gabapentin to help manage nerve pain resulting from the treatment.
Patients receive opioids 45 minutes to one hour before each infusion of Danyelza, and additional opioid treatments as needed. Some patients may additionally receive ketamine for any pain that is not adequately controlled by the opioids.
Half an hour before each administration of Danyelza, patients receive several medications to help reduce the chance of an reaction to the infusion:
- An antihistamine to prevent allergic reactions
- An H2 antagonist to prevent digestive problems
- Acetaminophen to prevent fever and pain
- An antiemetic to prevent nausea and vomiting
- Corticosteroids to prevent inflammation (if needed; given 2 hours to 30 minutes before Danyelza via a tube in the vein)
During administration of Danyelza and for 2 hours after the infusion, patients are closely monitored for reactions to the infusion. If symptoms of an infusion-related reaction occur, the infusion may be slowed or entirely stopped, depending on the severity of the reaction.
Treatment cycles are repeated every 4 weeks until a shrinkage or disappearance of the cancer. Five additional cycles are given every 4 weeks thereafter. Subsequent treatment cycles, if necessary, may be repeated every 8 weeks.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Neuroblastoma (previously treated)
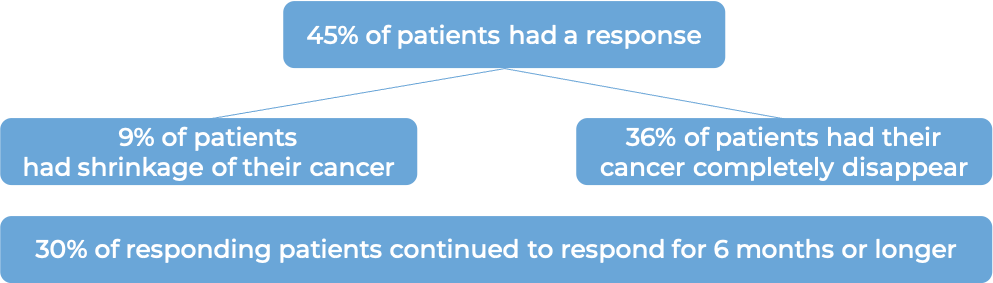
In a clinical trial, 22 patients (3 to 10 years old) with neuroblastoma in the bone or bone marrow who responded to a prior treatment, but whose cancer had since returned (relapsed) or stopped responding to the prior treatment (refractory), were treated with Danyelza in combination with GM-CSF.
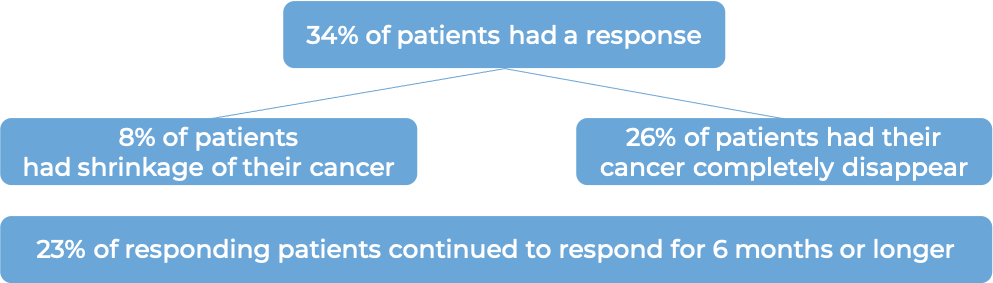
In another clinical trial, 38 patients (2-23 years old) with neuroblastoma in the bone or bone marrow who responded to a prior treatment, but whose cancer had since returned (relapsed) or stopped responding to the prior treatment (refractory), were treated with Danyelza in combination with GM-CSF.
What are the potential side effects?
The most common side effects of Danyelza include reactions related to the infusion, pain, increased heart rate, high blood pressure, “bullseye” rashes, nerve damage (tingling, numbness, or pain), hives, injection site reactions, swelling of the hands, ankles, or feet, anxiety, irritability, and abnormal blood test results. Cold- and flu-like symptoms such as coughing, headache, fatigue, fever, decreased appetite, nausea, vomiting, and diarrhea are also common.
Some side effects, such as infusion-related reactions, nerve damage, and high blood pressure can become serious or life-threatening. Patients and caregivers receive careful instructions to monitor for signs and symptoms related to these conditions. These conditions are managed by the healthcare provider.
Infusion-related reactions
Infusion-related reactions are an adverse response to receiving an infusion. Almost all patients treated with Danyelza experience infusion-related reactions with some level of severity. Most of these reactions typically occur within 24 hours of the first or second infusion. Symptoms include hives (itchy red welts) or rash, itchiness (pruritus), and swelling of the tongue, lips, face, or throat. Coughing, shortness of breath, wheezing, difficulty breathing, weakness, low blood pressure, dizziness, or faintness can also occur. Palpitations (feeling as if your heart is fluttering or racing) and chest pain can also be symptoms of a reaction. Serious reactions include anaphylactic shock and cardiac arrest (the heart stops pumping blood around the body).
Nerve damage
Because Danyelza can also target the GD2 molecule on nerve cells, treatment with Danyelza may result in damage to the nervous system, resulting in:
- Pain - almost all patients treated with Danyelza experienced bodily pain with some degree of severity, including pain in the stomach region, bones, neck, arms, and legs.
- Transverse myelitis - an inflammation of the spinal cord. Symptoms include pain, weakness, sensory problems (numbness, tingling, burning, or aversion to light, sound, touch, taste, or smell), and bladder or bowel problems.
- Reversible posterior leukoencephalopathy syndrome - caused by swelling in the brain that does not compromise blood flow. Symptoms of RPLS include high blood pressure, headache, seizures, visual disturbances, and altered consciousness.
- Peripheral neuropathy - caused by damage to nerves that are responsible for relaying sensory and motor information. Symptoms of peripheral neuropathy include pain, a persistent “pins and needles” sensation, tingling, chilling, burning, numbness, and weakness.
- Neurological disorders of the eye - Symptoms include unequal pupils, blurred or bad vision, difficulty focusing vision, dilated pupils, and extreme light sensitivity.
- Prolonged urinary retention - a condition in which patients cannot completely empty the bladder when peeing. If this condition does not resolve after the administration of opioids has been stopped, then treatment with Danyelza should be permanently stopped.
High blood pressure
High blood pressure is a common side effect of treatment with Danyelza, however in rare cases, the condition can become serious. Patients will have their blood pressure monitored during each infusion with Danyelza, and daily for the first 8 days of each treatment cycle, in order to ensure the patient does not experience dangerously high blood pressure.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. Treatment with Danyelza may be interrupted and later resumed at a lower dose, or discontinued entirely depending on the severity of the condition. For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer
Y-mAbs Therapeutics, Inc
Approval
FDA
Website
Last updated March 11, 2022


