How is the drug name pronounced?
Mogamulizumab: MOE-gah-MOO-liz-oo-MAB
Poteligeo: poh-teh-LIH-gee-oh
What cancer(s) does this drug treat?
Mycosis fungoides or Sézary syndrome
Poteligeo is approved for:
- Patients with Mycosis fungoides or Sézary syndrome who received at least one systemic therapy (treatment that affects the entire body), but whose cancer either did not respond to treatment (refractory), or has since returned (relapsed).
Limitations of Use
Age: The safety and efficacy of Poteligeo have not been established in patients under 18 years of age.
Pregnancy/Breastfeeding: Poteligeo may cause harm to the fetus and is not recommended for use during pregnancy. Women are advised to use contraception during treatment with Poteligeo and for at least 3 months after the last dose of Poteligeo. The risks associated with Poteligeo during breastfeeding are not known and cannot be ruled out. Due to the potential for serious side effects in the breastfed child, the benefits of breastfeeding and the mother’s need for treatment should be weighed accordingly before moving forward with treatment with Poteligeo.
What type of immunotherapy is this?
How does this drug work?
Target:
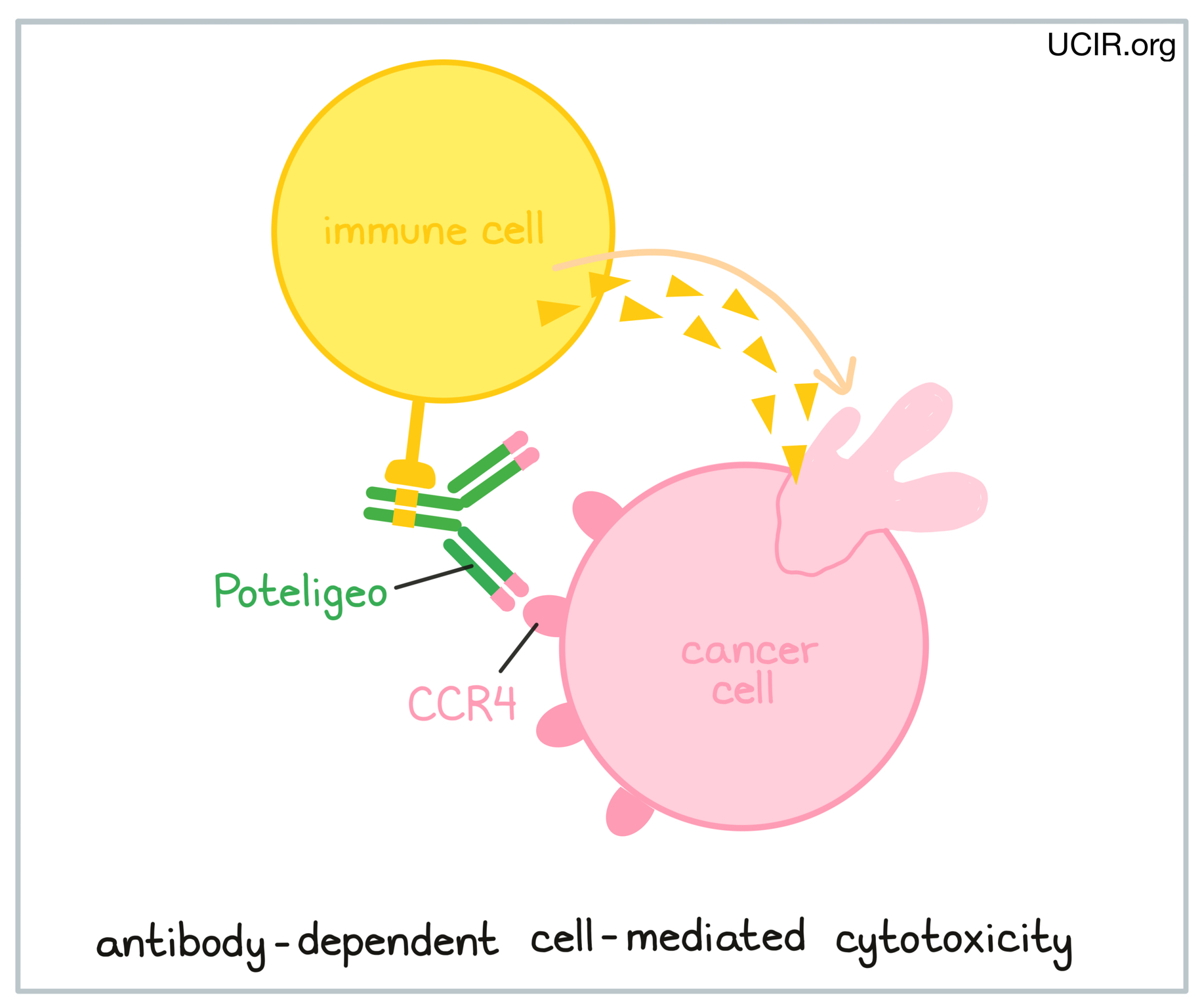
Poteligeo is an antibody that was made in a laboratory and designed to attach to a protein molecule called CCR4. CCR4 is present on the surface of some normal T cells, but is also found in high amounts on the surface of cancerous T cells in patients with Mycosis fungoides and Sézary syndrome. Higher-than-normal amounts of CCR4 on cancerous T cells make these cells the main target of Poteligeo.
Poteligeo and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to its target. For Poteligeo, the tips of the upper arms bind to CCR4. The stem of Poteligeo’s “Y” shape has binding sites for immune cells or other parts of the immune system.
Poteligeo works to kill cancer cells through antibody-dependent cell-mediated cytotoxicity (ADCC). When bound to CCR4 on the surface of cancerous or normal T cells, the stem of Poteligeo can also attract and bind immune cells (like NK cells). This allows Poteligeo to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell Poteligeo is bound to.
How is this drug given to the patient?
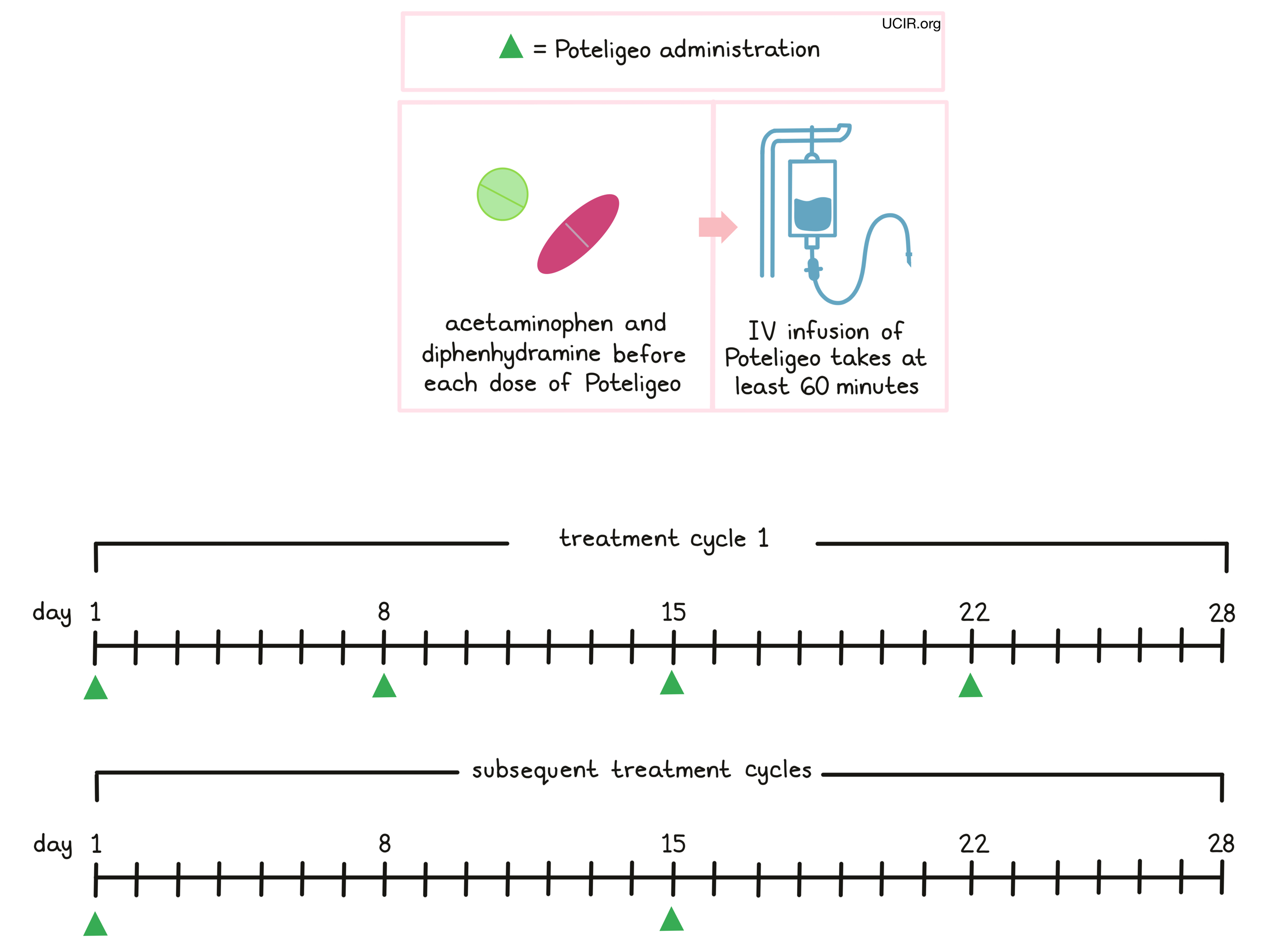
Poteligeo is administered via a tube in the vein (intravenous infusion, or i.v.) over at least 60 minutes once a week (on days 1, 8, 15, and 22) over the first 28 day (4 week) “treatment cycle”. In subsequent treatment cycles, Poteligeo is administered only once every two weeks, on days 1 and 15 of each cycle, until the disease progresses and gets worse, or the side effects become unmanageable.
Prior to the first infusion and subsequent infusions, if needed, patients receive acetaminophen and diphenhydramine in order to reduce the risk of reactions related to the infusion. During infusions, patients should be closely monitored for reactions. If a reaction occurs, treatment with Poteligeo may be interrupted and later resumed at a lower dose, or discontinued entirely depending on the severity of the reaction.
What are the observed clinical results?
It is important to keep in mind that each patient’s outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, it sometimes takes several months for responses to be observed.
Mycosis fungoides or Sézary syndrome
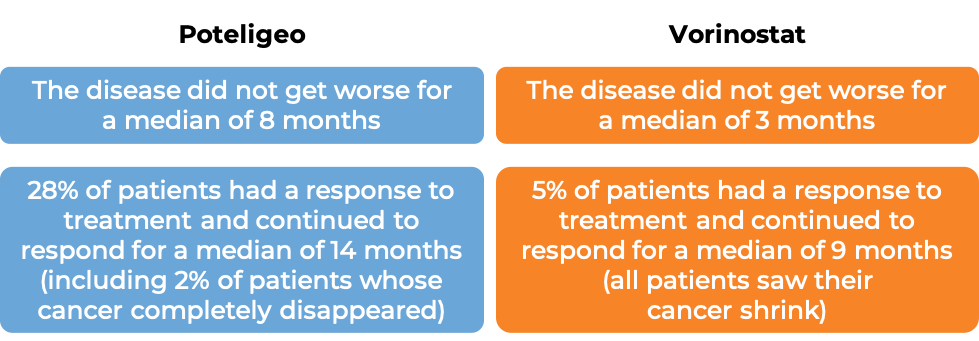
In a clinical trial, 372 patients with Mycosis fungoides or Sézary syndrome who had received at least one prior therapy, were either treated with Poteligeo or vorinostat.
The median duration of treatment for patients treated with Poteligeo was 6 months. For patients treated with vorinostat, the median duration of treatment was 3 months. At a median follow-up time of 13 months for patients treated with Poteligeo, and 10 months for patients treated with vorinostat:
What are the potential side effects?
The most common side effects of Poteligeo include rash, fatigue, diarrhea, upper respiratory tract infections, and pain in the muscles, bones, and/or ligaments.
Some side effects, such as skin reactions, reactions related to the infusion, infections, and autoimmune complications can become life-threatening, and may lead to death. Poteligeo may also cause complications with stem cell transplantation, even after discontinuing treatment. Patients and caregivers receive careful instructions to monitor for signs and symptoms of these complications. These conditions are managed by the healthcare provider.
Skin reactions
Poteligeo may cause serious and sometimes life-threatening skin reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Stevens-Johnson syndrome may initially present with flu-like symptoms, progressing into a widespread, blistering rash. As the condition progresses, the affected areas of the skin may begin to die and slough off. Toxic epidermal necrolysis is characterized by fever and reddening of the skin. The skin is often painful to the touch, and begins to blister and die, sloughing off.
Infusion-related reactions are negative responses to receiving an infusion. Infusion-related reactions typically occur within 24 hours of the first infusion, but can occur with subsequent infusions. Symptoms of an infusion-related reaction include hives (itchy red welts) or rash, itchiness, and swelling of the tongue, lips, face, or throat. Coughing, shortness of breath, wheezing, difficulty breathing, weakness, dizziness, or faintness can also occur. Palpitations (feeling as if your heart is fluttering or racing) and chest pain can also be symptoms of a reaction.
Infections
Patients taking Poteligeo are at increased risk of infections. Some of these infections can become serious, including sepsis, pneumonia, or skin infections.
Autoimmune complications
Poteligeo can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Poteligeo can cause side effects that may involve any organ, and can become serious or life-threatening. Some of these serious side effects include inflammation of the lungs, brain, liver (which can lead to liver failure), kidneys (which can lead to kidney failure), or colon. Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas), and nerves (which can lead to paralysis).
Complications of stem cell transplantation after taking Poteligeo
Poteligeo can cause serious and life-threatening complications in patients who receive a stem cell transplant from a donor after treatment with Poteligeo, especially if the transplant is given within 50 days after the last dose of Poteligeo. Such complications include graft-versus-host disease.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Kyowa Hakko Kirin Co., Ltd.
Approval
FDA and EMA
Website
Last updated August 11, 2025
How is the drug name pronounced?
Mogamulizumab: MOE-gah-MOO-liz-oo-MAB
Poteligeo: poh-teh-LIH-gee-oh
What cancer(s) does this drug treat?
Mycosis fungoides or Sézary syndrome
Poteligeo is approved for:
- Patients with Mycosis fungoides or Sézary syndrome who received at least one systemic therapy (treatment that affects the entire body).
Limitations of Use
Age: The safety and efficacy of Poteligeo have not been established in patients under 18 years of age.
Pregnancy/Breastfeeding: Poteligeo may cause harm to the fetus and is not recommended for use during pregnancy. Women are advised to use contraception during treatment with Poteligeo and for at least 6 months after the last dose of Poteligeo. The risks associated with Poteligeo during breastfeeding are not known and cannot be ruled out especially during the first few days after birth. Due to the potential for side effects in the breastfed child, women are advised not to breastfeed during the first few days after birth.
What type of immunotherapy is this?
How does this drug work?
Target:
Poteligeo is an antibody that was made in a laboratory and designed to attach to a protein molecule called CCR4. CCR4 is present on the surface of some normal T cells, but is also found in high amounts on the surface of cancerous T cells in patients with Mycosis fungoides and Sézary syndrome. Higher-than-normal amounts of CCR4 on cancerous T cells make these cells the main target of Poteligeo.
Poteligeo and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their target. For Poteligeo, the tips of the upper arms bind to CCR4. The stem of Poteligeo’s “Y” shape has binding sites for immune cells or other parts of the immune system.
Poteligeo works to kill cancer cells through antibody-dependent cell-mediated cytotoxicity (ADCC). When bound to CCR4 on the surface of cancerous or normal T cells, the stem of Poteligeo can also attract and bind immune cells (like NK cells). This allows Poteligeo to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell Poteligeo is bound to.

How is this drug given to the patient?
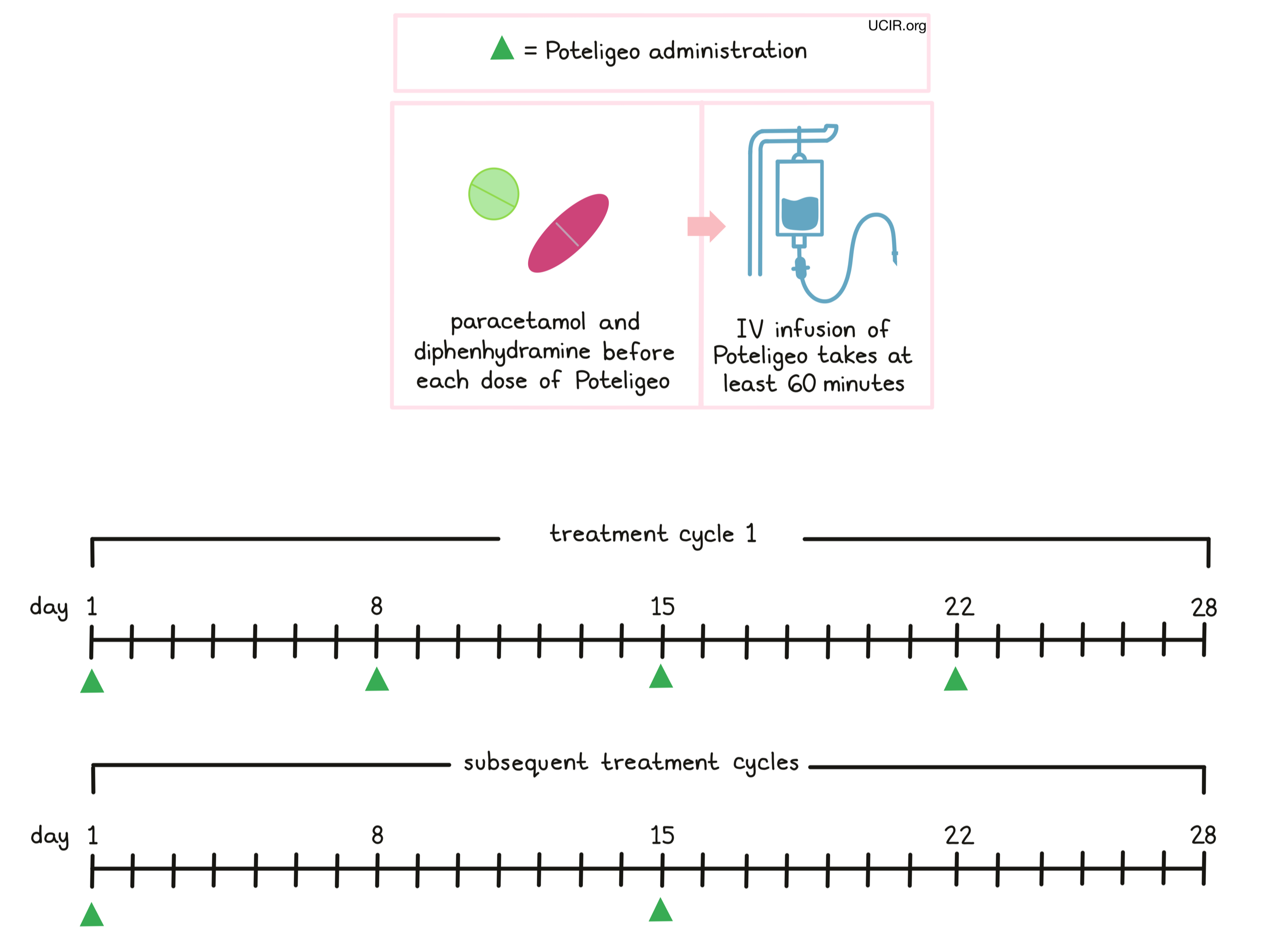
Poteligeo is administered via a tube in the vein (intravenous infusion, or i.v.) over at least 60 minutes once a week (on days 1, 8, 15, and 22) over the first 28 day (4 week) “treatment cycle”. In subsequent treatment cycles, Poteligeo is administered only once every two weeks, on days 1 and 15 of each cycle, until the disease progresses and gets worse, or the side effects become unmanageable.
Prior to the first infusion and subsequent infusions, if needed, patients receive paracetamol and diphenhydramine in order to reduce the risk of reactions related to the infusion. During infusions, patients should be closely monitored for reactions. If a reaction occurs, treatment with Poteligeo may be interrupted and later resumed at a lower dose, or discontinued entirely depending on the severity of the reaction.
What are the observed clinical results?
It is important to keep in mind that each patient’s outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, it sometimes takes several months for responses to be observed.
Mycosis fungoides or Sézary syndrome
In a clinical trial, 372 patients with Mycosis fungoides or Sézary syndrome who had received at least one prior therapy, were either treated with Poteligeo or vorinostat.
The median duration of treatment for patients treated with Poteligeo was 6 months. For patients treated with vorinostat, the median duration of treatment was 3 months. At a median follow-up time of 13 months for patients treated with Poteligeo, and 10 months for patients treated with vorinostat:

What are the potential side effects?
The most common side effects of Poteligeo include rash, fatigue, diarrhea, upper respiratory tract infections, and pain in the muscles, bones, and/or ligaments.
Some side effects, such as skin reactions, reactions related to the infusion, infections, autoimmune complications, problems with the heart, and tumor lysis syndrome (a condition caused by the rapid breakdown of cancer cells) can become serious, life-threatening, and may lead to death. Poteligeo may also cause complications with stem cell transplantation, even after discontinuing treatment. Patients and caregivers receive careful instructions to monitor for signs and symptoms of these complications. These conditions are managed by the healthcare provider.
Skin reactions
Poteligeo may cause serious and sometimes life-threatening skin reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Stevens-Johnson syndrome may initially present with flu-like symptoms, progressing into a widespread, blistering rash. As the condition progresses, the affected areas of the skin may begin to die and slough off. Toxic epidermal necrolysis is characterized by fever and reddening of the skin. The skin is often painful to the touch, and begins to blister and die, sloughing off.
Infusion-related reactions are a negative response to receiving an infusion. Infusion-related reactions typically occur within 24 hours of the first infusion, but can occur with subsequent infusions. Symptoms of an infusion-related reaction include hives (itchy red welts) or rash, itchiness, and swelling of the tongue, lips, face, or throat. Coughing, shortness of breath, wheezing, difficulty breathing, weakness, dizziness, or faintness can also occur. Palpitations (feeling as if your heart is fluttering or racing) and chest pain can also be symptoms of a reaction.
Infections
Patients taking Poteligeo are at increased risk of infections. Some of these infections can become serious, including sepsis, pneumonia, or skin infections. Patients will be screened for the hepatitis B virus prior to Poteligeo treatment. In patients who had hepatitis B in the past, or who are carriers of the hepatitis B virus, Poteligeo can cause the virus to become active again. These patients will need to be monitored closely during treatment and for several months after Poteligeo treatment.
Autoimmune complications
Poteligeo can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Poteligeo can cause side effects that may involve any organ, and can become serious or life-threatening. Some of these serious side effects include inflammation of the lungs, brain, liver (which can lead to liver failure), kidneys (which can lead to kidney failure), or colon. Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas), and nerves (which can lead to paralysis).
Complications of stem cell transplantation after taking Poteligeo
Poteligeo can cause serious and life-threatening complications in patients who receive a stem cell transplant from a donor after treatment with Poteligeo, especially if the transplant is given within 50 days after the last dose of Poteligeo. Such complications include graft-versus-host disease.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Kyowa Hakko Kirin Co., Ltd.
Approval
FDA and EMA
Website
Last updated on August 11, 2025






