How is the drug name pronounced?
Mirvetuximab soravtansine: MEER-veh-TUK-sih-mab SOH-rav-TAN-seen
Elahere: el-ah-HERE
What cancer(s) does this drug treat?
Advanced ovarian, fallopian tube, or primary peritoneal cancer
Elahere is approved for:
-
Patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer:
- whose cancer tests positive for folate receptor alpha (FRα), AND
- whose disease is resistant to chemotherapy containing platinum (disease initially responded to treatment, but came back within 6 months), AND
- who have received up to three prior therapies for their disease.
Limitations of Use
Age: The safety and efficacy of Elahere have not been established in patients under 18 years of age.
Fertility/Pregnancy/breastfeeding: Elahere can cause harm to the fetus and is not recommended for use during pregnancy. Patients are advised to use contraception during treatment with Elahere and for at least 7 months after the last dose of Elahere. The risks associated with Elahere during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, patients are advised not to breastfeed during treatment with Elahere and for up to 1 month after the last dose of Elahere.
What type of immunotherapy is this?
- Antibody–drug conjugate
How does this drug work?
Target:
- Folate receptor alpha (FRα)
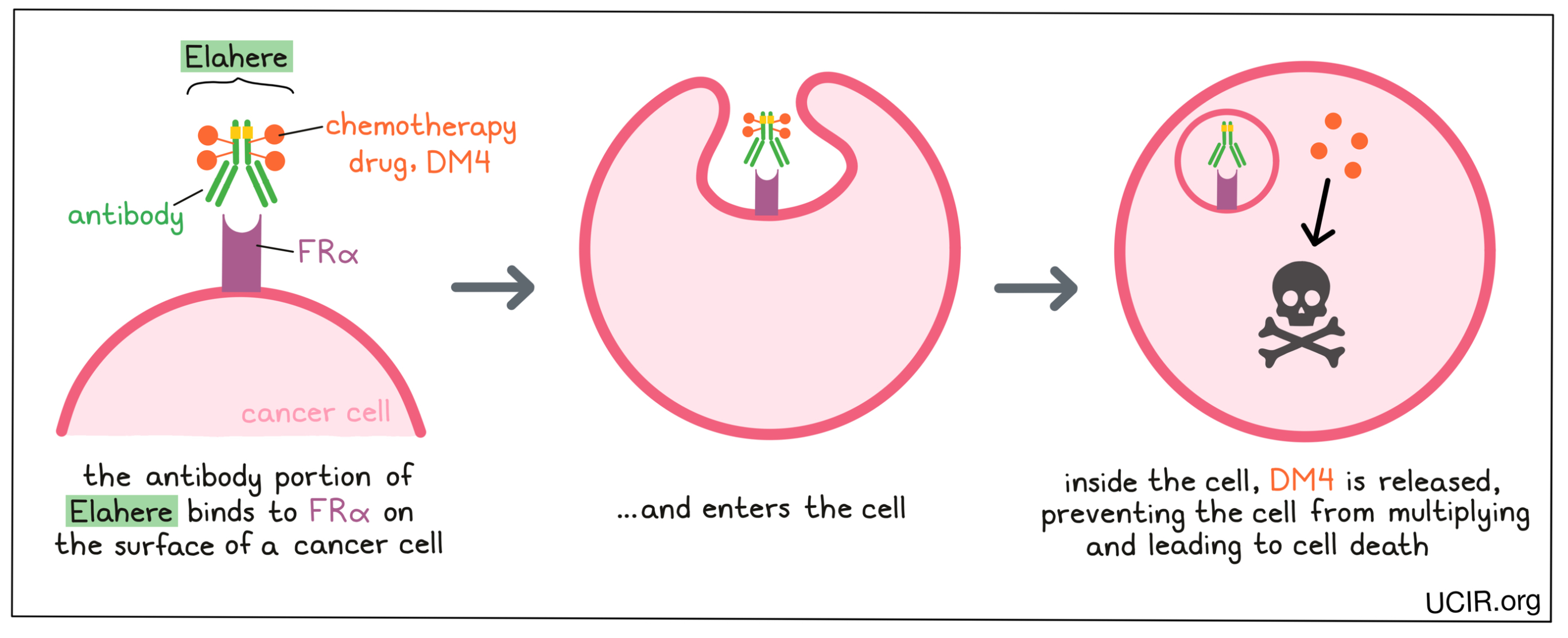
Elahere is a medicine that consists of two parts that are connected:
- an antibody that attaches to a molecule called folate receptor alpha (FRα) on the surface of cancer cells (and some healthy cells), AND
- a chemotherapy drug called DM4
Elahere and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets, in this case, FRα. The stem of Elahere’s “Y” shape has the chemotherapy drug DM4 connected to it.
When Elahere binds to FRα, it can enter the cell it is bound to. Inside the cell, the chemotherapy part of Elahere is released and becomes activated. DM4 prevents the cancer cells from multiplying and leads to their death. By attaching to FRα, which is present in high amounts on cancer cells, Elahere is designed to minimize harm to normal, healthy cells, and to bring the chemotherapy directly to the cancer cells.
How is this drug given to the patient?
Elahere is administered through a tube in the vein (intravenous infusion, or i.v.) over 30 minutes once every 3 weeks.
Prior to receiving each dose of Elahere, patients receive several medications to help reduce the risk and severity of reactions related to the infusion:
- A corticosteroid (dexamethasone or methylprednisolone)
- An antihistamine (diphenhydramine)
- A painkiller and fever reducer (acetaminophen)
- An antiemetic (to prevent nausea and vomiting)
Due to the risk of damage to the eyes, patients should have an eye examination before starting treatment with Elahere, prior to every other dose of Elahere, and promptly if eye problems arise. Treatment with Elahere may be withheld or permanently stopped based on the severity and potential improvement of the eye problems. Starting the day prior to each Elahere infusion, on the day of the infusion and for 3 days thereafter, patients should administer one drop of eye drops containing corticosteroids into each eye 6 times daily. On days 5 to 8, drops should be administered 4 times daily. Patients are also advised to use preservative-free lubricant eye drops at least 4 times a day and to avoid the use of contact lenses during treatment with Elahere. The lubricant eye drops should be administered at least 10 minutes after the administration of the corticosteroid eye drops.
Prior to starting Elahere treatment, patients also get blood work done.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies described below. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced ovarian, fallopian tube, or primary peritoneal cancer
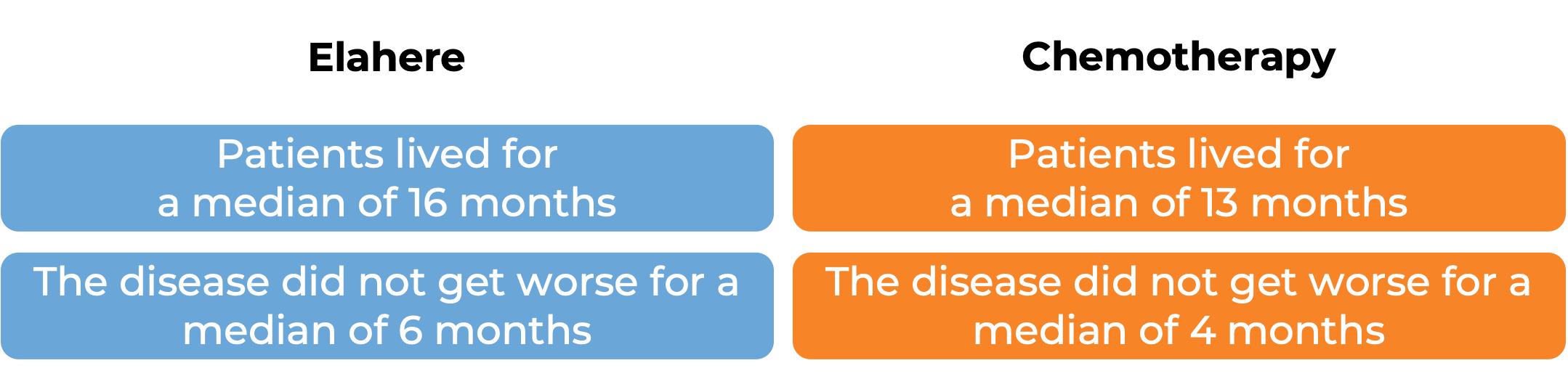
In a clinical trial, 453 patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer that tested positive for the FRα molecule and did not respond to prior treatment with a platinum-based chemotherapy, were treated with Elahere or chemotherapy.
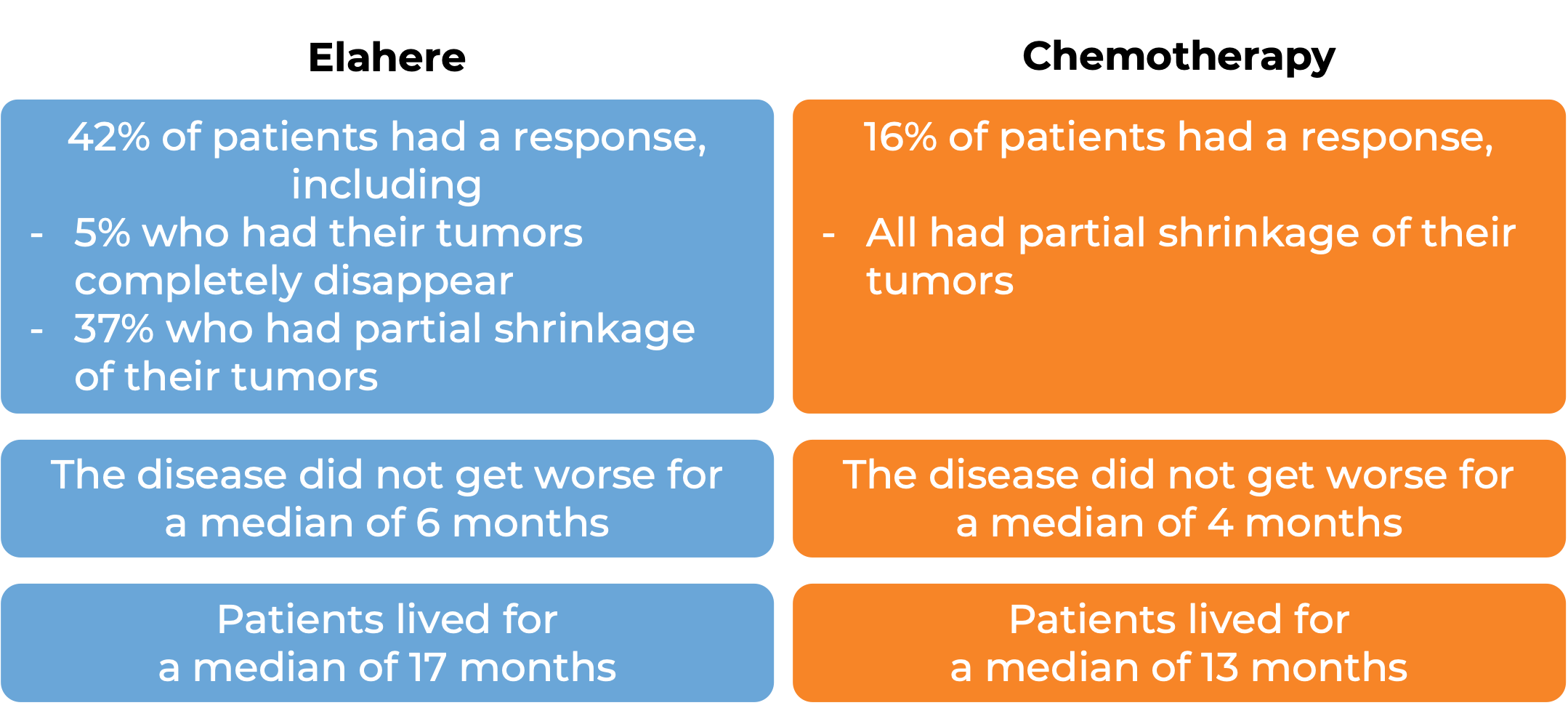
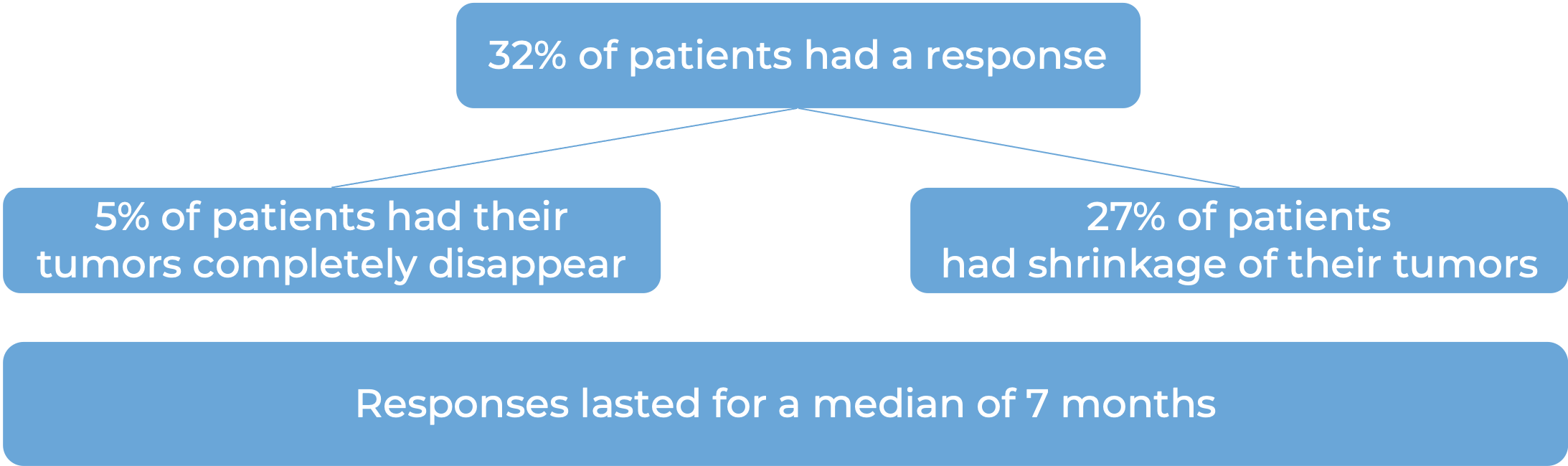
In a clinical trial, 104 patients with previously treated epithelial ovarian, fallopian tube, or primary peritoneal cancer that tested positive for folate receptor alpha (FRα) and was resistant to chemotherapy containing platinum, were treated with Elahere.
(For the definition of “median”, click HERE.)
What are the potential side effects?
The most common side effects with Elahere include eye problems, nausea, fatigue, reactions related to Elahere infusion, diarrhea, constipation, abdominal pain, numbness or tingling in the hands or feet, muscular weakness, low white blood cell count, and other abnormal blood test results.
Eye problems
Elahere can damage the corneal epithelium, the clear layer of tissue covering the pupil and iris, or cause uveitis. This can result in dry eyes, discomfort in bright light, corneal ulcers, blurry vision, worsening vision, and vision loss. Eye exams are conducted prior to and during treatment in order to assess whether any corneal damage has taken place. If problems arise, the administration of Elahere is interrupted until the symptoms improve, or is permanently stopped.
For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer
AbbVie
Approval
FDA and EMA
Link to drug website
Last updated: November 18, 2025