How is this drug name pronounced?
Talimogene laherparepvec (T-Vec): tal-IM-oh-jeen la-HER-pa-REP-vek
Imlygic: im-LY-jik
What cancer(s) does this drug treat?
Recurrent melanoma that cannot be removed by surgery
Imlygic is approved for:
- Patients with melanoma that has recurred after initial surgery and cannot be surgically removed again. Patients must have tumors on the skin, in the layers below the skin, or in lymph nodes that can be located by touch or by ultrasound.
Limitations of use
Age: Safety and effectiveness of Imlygic have not been established in pediatric patients.
Exclusions: Imlygic is a live, but weakened herpes simplex virus and may cause life-threatening viral infections in patients with a weakened immune system. Thus, Imlygic should not be given to immunocompromised patients (those with a history of primary or acquired immunodeficient states, leukemia, lymphoma, AIDS or symptoms of HIV infection, or those on immunosuppressive therapy). Imlygic should not be injected into the liver.
Pregnancy/Breastfeeding/Fertility: In pregnant women infected by wild type (unmodified) herpes simplex virus Type 1, the virus may potentially cross the placental barrier and infect the fetus, or may infect the fetus during childbirth, leading to potentially life-threatening consequences for the fetus. Therefore, Imlygic should not be used in pregnant patients. Women of childbearing potential should be advised to use an effective method of contraception to prevent pregnancy during treatment with Imlygic.
There is no information regarding the presence of Imlygic in human milk. Lactating patients treated with Imlygic should consult their doctor regarding whether to continue breastfeeding during treatment.
There is no information on the impact of Imlygic treatment on fertility.
What type of immunotherapy is this?
How does this drug work?
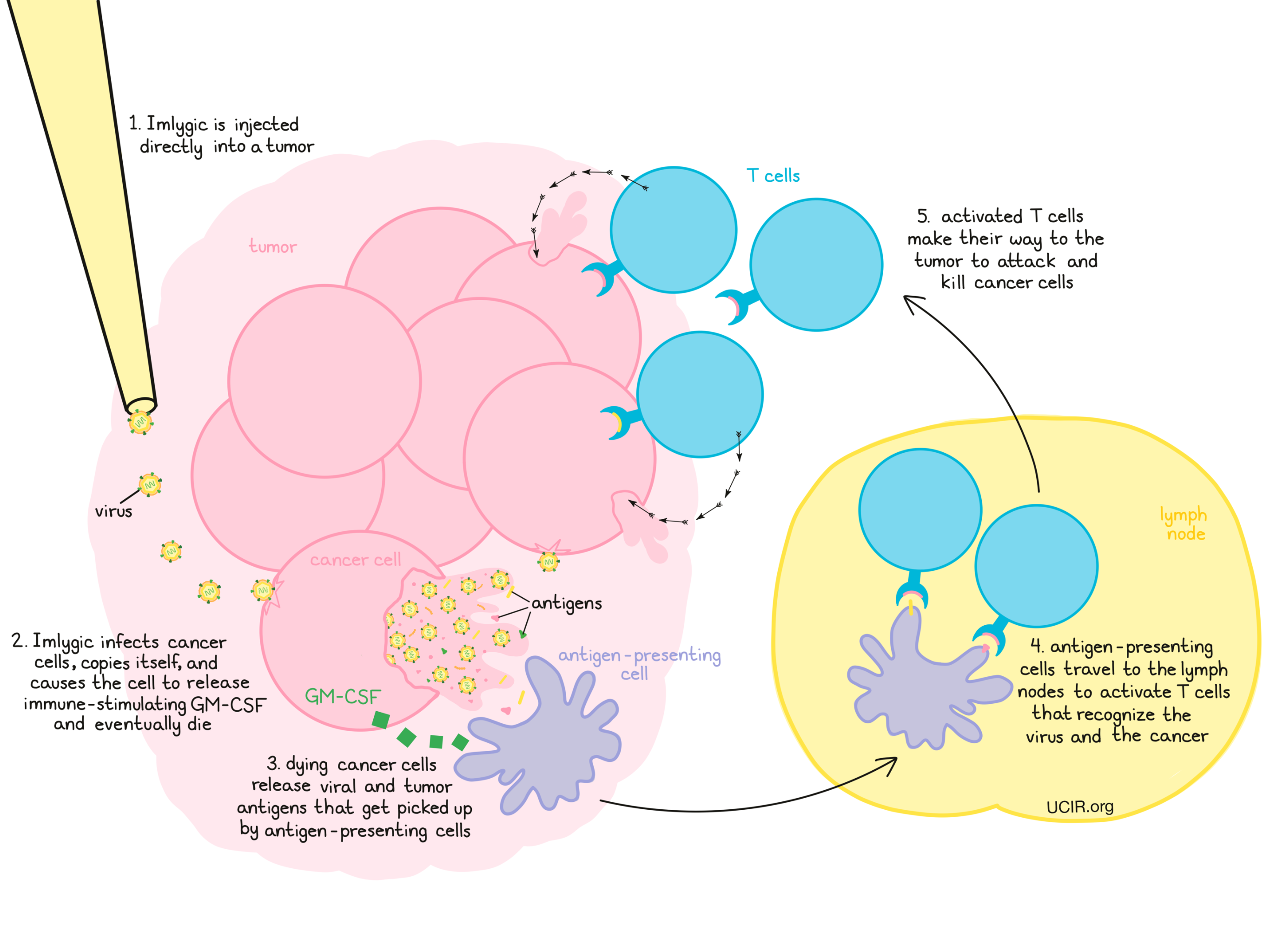
Imlygic is a modified Herpes virus that preferentially infects and kills cancer cells compared to normal cells. Imlygic was modified by deletion of two genes, which resulted in a virus that more effectively grows in and kills cancer cells. Additionally, the gene for Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) was added to the virus. After the virus infects of cancer cells, it releases its genetic material and instructs the cancer cells to produce new copies of the virus and to produce and release GM-CSF. GM-CSF is a molecule that promotes the initiation of an immune response against the cancer cells. Eventually, cancer cells infected by the virus will die, releasing more copies of the virus, along with cellular debris.
The virus infection itself, in addition to the GM-CSF, help recruit the patient’s immune cells to the injected tumor site. Debris from killed cancer cells contains viral and tumor antigens that get picked up by antigen-presenting cells. Antigen-presenting cells then travel to the lymph nodes where they activate T cells that recognize both the virus and the cancer as “foreign”. Activated T cells then make their way to the tumor, where they attack and kill cancerous cells and cells infected by Imlygic. The activated T cells can also circulate throughout the patient’s body to attack and kill cancer cells that may have spread to other sites.
How is this drug given to the patient?
Using a syringe and needle, Imlygic is injected directly into tumors that are on or under the skin or in lymph nodes that can be located by touch or by ultrasound. Just prior to treatment, a local anesthetic may be applied or injected to numb the injection site. The largest tumor is treated first, and then any additional tumors may be treated until the full dose is delivered. The volume of Imlygic delivered per tumor is based on the tumor size. If new lesions appear between treatments, the new lesions are injected first at the next visit.
The needle containing Imlygic is usually inserted through the skin only once in each treated tumor, but the needle will be slightly pulled back and moved to different locations in the tumor so that Imlygic can be delivered throughout the entire tumor mass. In very large tumors, a separate injection may be needed.
The injection site should remain covered for at least a week, or for longer if the injection is weeping or oozing.
The first dose of Imlygic contains a low number of viral particles (only 1% of the number of particles used for all subsequent doses). A second dose is delivered three weeks later, and then every two weeks thereafter for at least 6 months, or until other treatment is required, or until lesions disappear.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies.
Recurrent melanoma that cannot be removed by surgery
In a large clinical study involving patients with advanced melanoma that could not be removed by surgery, but had a least one tumor site that was accessible for direct or ultrasound-guided injection, patients were either treated with Imglytic or with GM-CSF (the immune-stimulating molecule that is produced and released by cancer cells upon infection with Imlygic). At a median follow-up of 3.5 years:
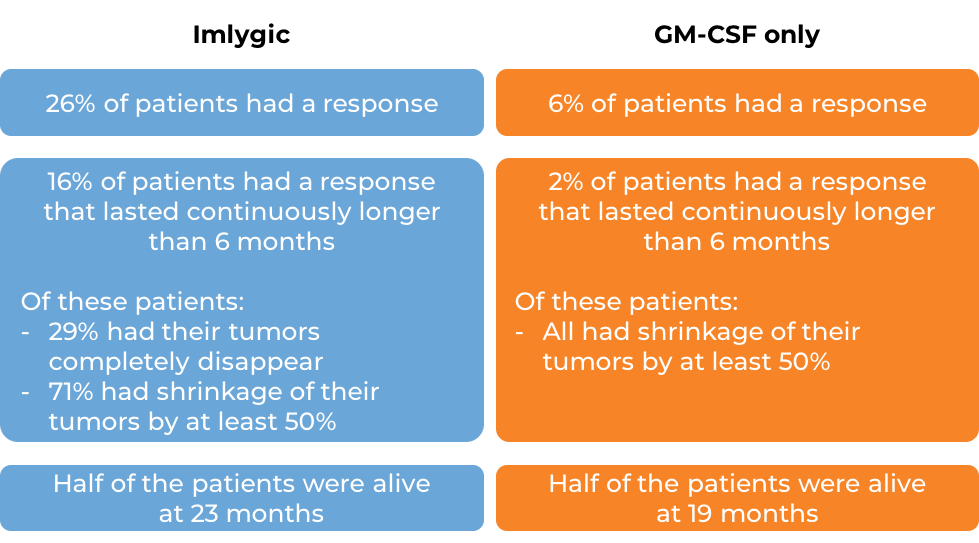
Tumor shrinkage in response to treatment with Imlygic occurred in half of the patients by 4 months after starting therapy. Some patients responded as early as 1 month after initial treatment. Others responded as late as 17 months. More clinical trials are ongoing.
What are the side effects?
The most commonly reported adverse drug reactions were fatigue, chills, fever, nausea, influenza-like illness, and pain at the injection site. One or more of these symptoms occurred in more than 25-50% of patients.
Herpes virus-caused cold sores, inflammation in and around the eyes, and other signs of herpes infection have been reported; patients should report these symptoms to their health care provider for evaluation. Spreading herpes virus infection may also occur in immunocompromised patients.
Tissue death or ulceration of tumor tissue may occur. Impaired healing of the injection site, infections just below the skin (cellulitis), and infections elsewhere in the body have also been reported.
Because Imlygic is a live, replicating virus, the virus can be transmitted to others through contact with injected lesions or bodily fluids. Accidentally exposed individuals should wash the area of contact well and inform their healthcare professional if any potential symptoms of a viral infection arise.
Additional information
Manufacturer
Amgen
Approval
FDA and EMA
Link to patient information
Links to drug websites
- US: https://www.fda.gov/vaccines-blood- biologics/cellular-gene-therapy-products/imlygic-talimogene-laherparepvec
- Europe: https://www.ema.europa.eu/en/medicines/human/EPAR/imlygic
Other references
- Talimogene Laherparepvec Improves Durable Response Rates in Patients with Advanced Melanoma. Andtbacka RHI, Kaufman HL, Collichio F, et al Journal of Clinical Oncology (2015).
- Talimogene laherparepvec: review of its mechanism of action and clinical efficacy and safety. Raman SS, Hecht JR, Chan E. Immunotherapy (2019).
Last updated January 16, 2023


