How is this drug name pronounced?
Isatuximab: I-suh-TUK-sih-mab
Sarclisa: sar-KLIH-suh
What cancer(s) does this drug treat?
Multiple myeloma
Sarclisa is approved for:
- Patients with multiple myeloma who have received at least two prior treatments for their disease, including lenalidomide (Revlimid) and a proteasome inhibitor. In such cases, Sarclisa is used in combination with pomalidomide (Pomalyst) and dexamethasone.
- Patients with multiple myeloma who have received up to three prior treatments for their disease, and these either did not work or stopped working and the cancer has since come back. In such cases, Sarclisa is used in combination with carfilzomib and dexamethasone.
Limitations of use:
Age: The safety and efficacy of Sarclisa in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Sarclisa may cause harm to a fetus, and is not recommended for use during pregnancy. Women should not start Sarclisa treatment when pregnant and are advised to use two methods of reliable contraception during treatment with Sarclisa and for at least 5 months after the last dose of Sarclisa. Men are advised to use contraception during treatment with Sarclisa and for at least 4 weeks after the last dose of pomalidomide (Pomalyst) as part of the Sarclisa treatment. The risks associated with Sarclisa during breastfeeding are not known and cannot be ruled out. Due to the potential for adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Sarclisa.
What type of immunotherapy is this?
How does this drug work?
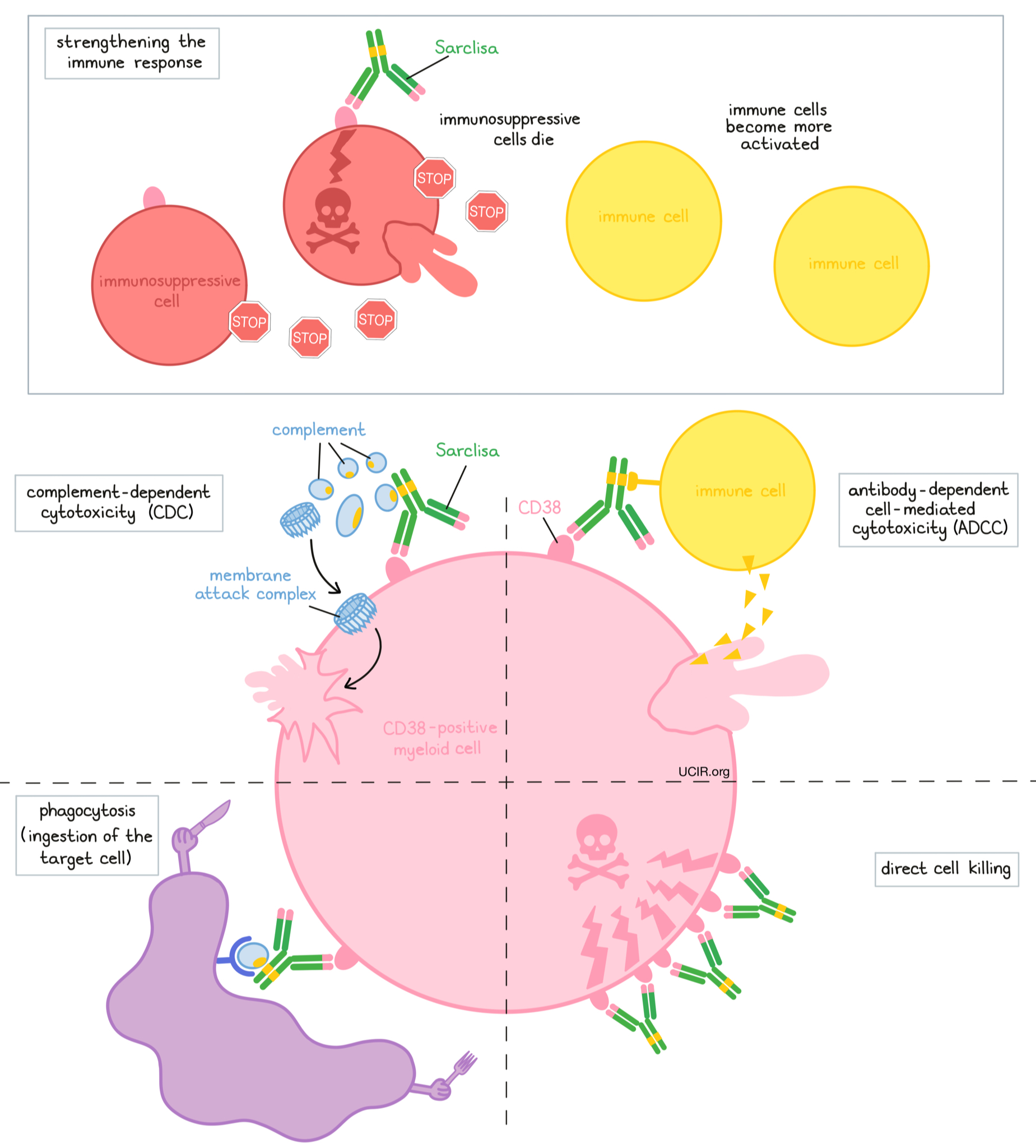
Sarclisa is an antibody that was made in the laboratory. Sarclisa and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. Sarclisa attaches to a molecule called CD38. CD38 plays a role in the attachment of neighboring cells to each other, and in transmitting messages from outside of the cell to the inside of the cell. CD38 is present on the surface of many types of immune cells (white blood cells) and red blood cells, and is found in high amounts on the surface of myeloma cells.
The stem of Sarclisa’s “Y” shape has binding sites for immune cells or other parts of the immune system.
At least five different mechanisms are thought to be responsible for the elimination of myeloma cells by Sarclisa. Sarclisa works alone and/or is helped by the immune system to kill myeloma cells.
Direct cell killing
By binding to CD38 molecules on the surface of myeloma cells, Sarclisa can directly cause the cells to die.
Complement-dependent cytotoxicity (CDC)
When bound to CD38 on the surface of myeloma cells, the “stem” of Sarclisa can attract and bind molecules of the immune system called “complement” that freely flow in the blood or tissues. Activation of the complement system causes the formation of the “membrane attack complex”, which can puncture and destroy the cell that Sarclisa is bound to.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to CD38 on the surface of myeloma cells, the “stem” of Sarclisa can also attract and bind immune cells. This allows Sarclisa to act as a bridge between the target cell and the immune cell (such as the natural killer (NK) cell). The immune cell then releases molecules that can kill the cell that Sarclisa is bound to.
Phagocytosis
When Sarclisa is bound to myeloma cells, it can also attract immune cells called phagocytes, which have the ability to ingest or “eat” cells that have been coated with Sarclisa.
Strengthening the immune response
Sarclisa can bind to CD38 on the surface of certain types of immunosuppressive immune cells that reduce the strength of an immune response, causing such cells to die. At the same time, Sarclisa can activate other immune cells that are required for the elimination of cancer cells, thus allowing for a stronger immune attack on myeloma cells.
The combined effect of these mechanisms results in the elimination of melanoma from the body. Sarclisa is administered in combination with pomalidomide (Pomalyst). Pomalidomide has been shown to improve Sarclisa’s ability to destroy myeloma cells. Because CD38 is also found on certain normal cells, Sarclisa can attack and reduce the numbers of some types of immune cells.
How is this drug given to the patient?
Approximately 15 to 60 minutes prior to each administration of Sarclisa, patients receive several medications to help reduce the chance of a reaction to the infusion:
- An H1 blocker antihistamine (diphenhydramine)
- An H2 blocker antihistamine (ranitidine)
- A painkiller and fever reducer (acetaminophen)
- A corticosteroid (dexamethasone)
Sarclisa is administered via a tube into a vein (intravenous infusion, or I.V.) several times during a 28-day “treatment cycle”. During the first treatment cycle, Sarclisa is administered weekly on days 1, 8, 15, and 22. During the subsequent cycles, Sarclisa is administered every two weeks, on days 1 and 15. Sarclisa is administered in combination with pomalidomide (Pomalyst) and dexamethasone or in combination with carfilzomib and dexamethasone. Pomalidomide is taken by mouth once daily from day 1 to day 21 of each 28-day treatment cycle. Carfilzomib is administered by injection in a vein on days 1, 2, 8, 9, 15, and 16 of each 28-day treatment cycle. Low-dose dexamethasone (in addition to the corticosteroid that is injected into a vein before Sarclisa infusion) is given on days 1, 8, 15, and 22 for each 28-day cycle (either by mouth or by injection into a vein). On the days when both Sarclisa and carfilzomib are administered, dexamethasone is given first, followed by the Sarclisa, and then the carfilzomib infusion.
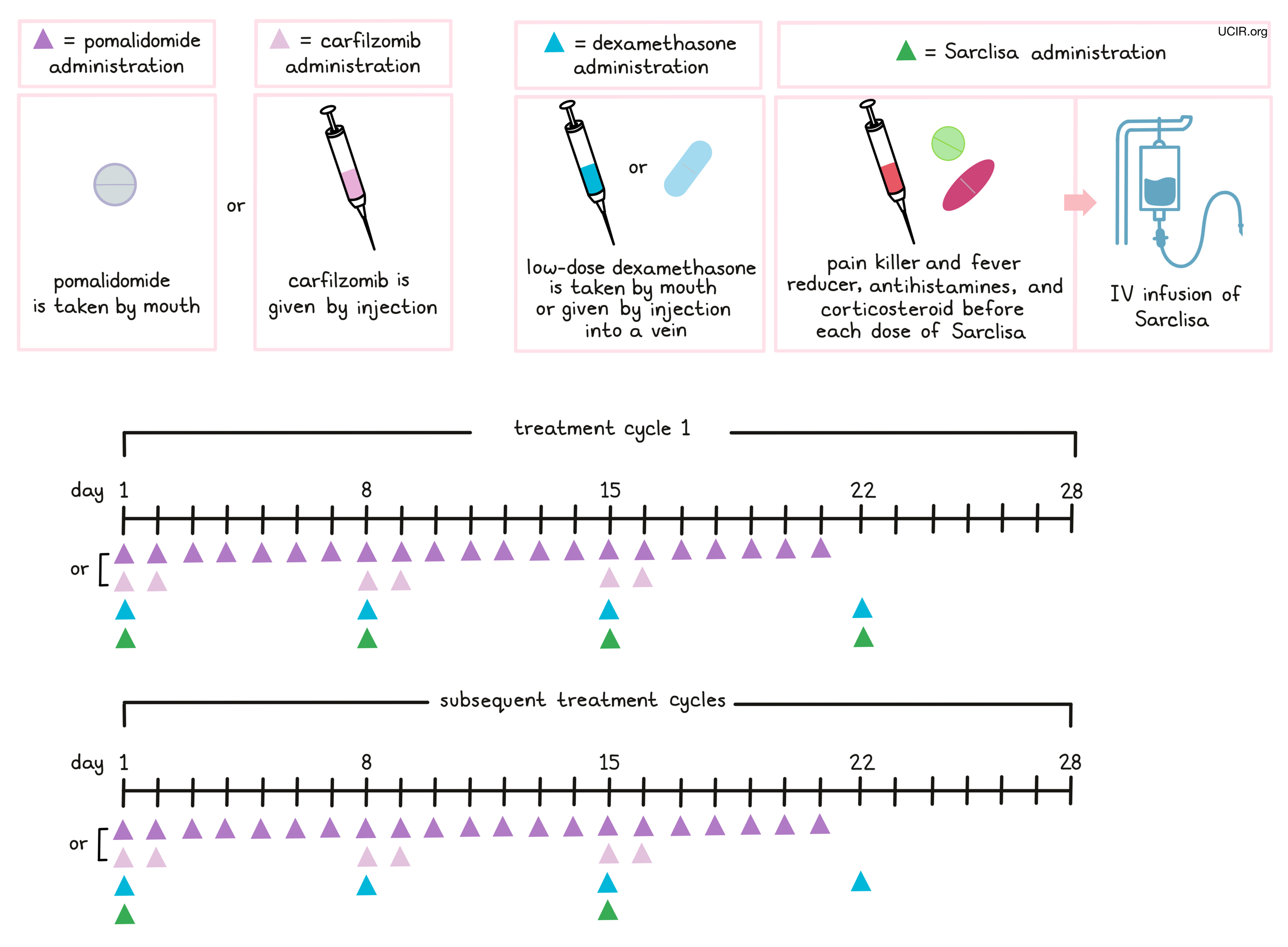
Patients may also receive antiviral or antibiotic medication to prevent herpes zoster (shingles) reactivation or infections during treatment with Sarclisa.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Multiple myeloma
In a clinical trial, 307 patients with multiple myeloma who had received prior treatment for their disease, and the treatment either did not work or stopped working, were treated with either:
- Sarclisa, pomalidomide (Pomalyst), and low-dose dexamethasone, OR
- pomalidomide and low-dose dexamethasone
At a median follow-up of 12 months:
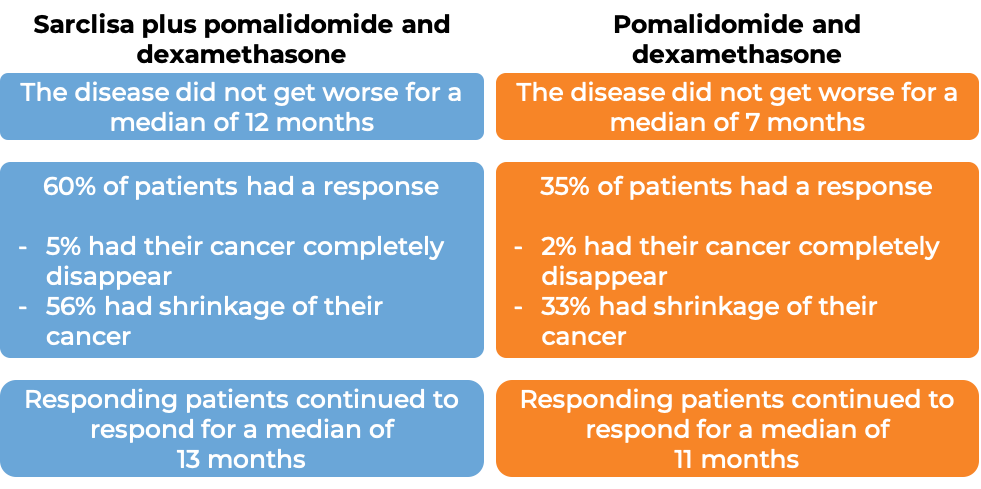
(For the definition of “median”, click HERE.)
In another clinical trial, 302 patients with multiple myeloma who had received up to three prior treatments for their disease, and the treatment either did not work or stopped working, were treated with either:
- Sarclisa, carfilzomib, and dexamethasone OR
- carfilzomib and dexamethasone.
At a median follow-up of 21 months:
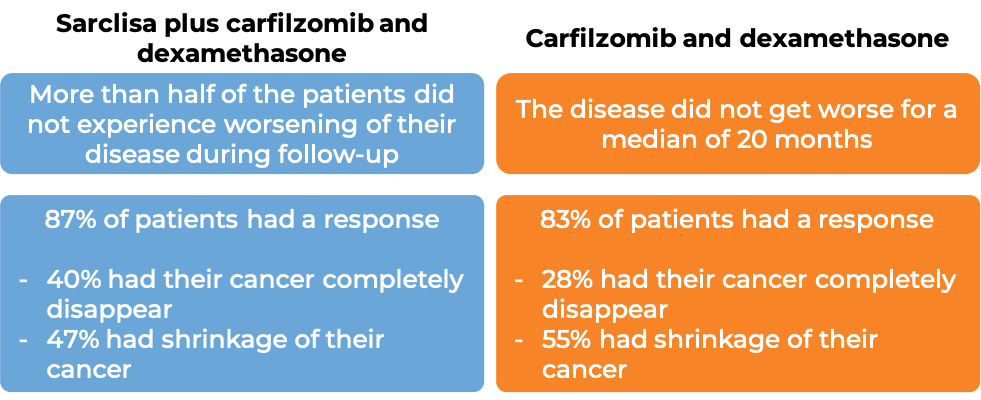
What are the side effects?
The most common side effects of Sarclisa include reactions related to the infusion (such as cough, chills, nausea, difficulty breathing, and high blood pressure), low white blood cell count (which can lead to serious infections), lung infection (pneumonia), upper respiratory tract infection, difficulty breathing, diarrhea, nausea, vomiting, low red blood cell count, and low platelet count. Sarclisa may interfere with the results of blood tests that are needed before a patient can receive a blood transfusion. Tests to match a patient’s blood type should be done before the first dose of Sarclisa. Sarclisa can increase the risk of developing new cancers (including skin cancer).
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Sanofi Aventis
Approval
FDA and EMA
Links to drug websites
Last updated: May 2024
How is this drug name pronounced?
Isatuximab: I-suh-TUK-sih-mab
Sarclisa: sar-KLIH-suh
What cancer(s) does this drug treat?
Multiple myeloma
Sarclisa is approved for:
- Patients with multiple myeloma who have received at least two prior treatments for their disease, including lenalidomide (Revlimid) and a proteasome inhibitor, and the treatment either did not work or stopped working. In such cases, Sarclisa is used in combination with pomalidomide (Pomalyst) and dexamethasone.
- Patients with multiple myeloma who have received at least one prior treatment for their disease, and it either did not work or stopped working and the cancer has since come back. In such cases, Sarclisa is used in combination with carfilzomib and dexamethasone.
Limitations of use:
Age: The safety and efficacy of Sarclisa in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Sarclisa may cause harm to a fetus, and is not recommended for use during pregnancy. Women should not start Sarclisa treatment when pregnant and are advised to use two methods of reliable contraception during treatment with Sarclisa and for at least 5 months after the last dose of Sarclisa. Men are advised to use contraception during treatment with Sarclisa and for at least 4 weeks after the last dose of pomalidomide (Pomalyst) as part of the Sarclisa treatment. The risks associated with Sarclisa during breastfeeding are not known and cannot be ruled out. Due to the potential for adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Sarclisa.
What type of immunotherapy is this?
How does this drug work?
Sarclisa is an antibody that was made in the laboratory. Sarclisa and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. Sarclisa attaches to a molecule called CD38. CD38 plays a role in the attachment of neighboring cells to each other, and in transmitting messages from outside of the cell to the inside of the cell. CD38 is present on the surface of many types of immune cells (white blood cells) and red blood cells, and is found in high amounts on the surface of myeloma cells.
The stem of Sarclisa’s “Y” shape has binding sites for immune cells or other parts of the immune system.
At least five different mechanisms are thought to be responsible for the elimination of myeloma cells by Sarclisa. Sarclisa works alone and/or is helped by the immune system to kill myeloma cells.
Direct cell killing
By binding to CD38 molecules on the surface of myeloma cells, Sarclisa can directly cause the cells to die.
Complement-dependent cytotoxicity (CDC)
When bound to CD38 on the surface of myeloma cells, the “stem” of Sarclisa can attract and bind molecules of the immune system called “complement” that freely flow in the blood or tissues. Activation of the complement system causes the formation of the “membrane attack complex”, which can puncture and destroy the cell that Sarclisa is bound to.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to CD38 on the surface of myeloma cells, the “stem” of Sarclisa can also attract and bind immune cells. This allows Sarclisa to act as a bridge between the target cell and the immune cell (such as the natural killer (NK) cell). The immune cell then releases molecules that can kill the cell that Sarclisa is bound to.
Phagocytosis
When Sarclisa is bound to myeloma cells, it can also attract immune cells called phagocytes, which have the ability to ingest or “eat” cells that have been coated with Sarclisa.
Strengthening the immune response
Sarclisa can bind to CD38 on the surface of certain types of immunosuppressive immune cells that reduce the strength of an immune response, causing such cells to die. At the same time, Sarclisa can activate other immune cells that are required for the elimination of cancer cells, thus allowing for a stronger immune attack on myeloma cells.
The combined effect of these mechanisms results in the elimination of melanoma from the body. Sarclisa is administered in combination with pomalidomide (Pomalyst). Pomalidomide has been shown to improve Sarclisa’s ability to destroy myeloma cells. Because CD38 is also found on certain normal cells, Sarclisa can attack and reduce the numbers of some types of immune cells.

How is this drug given to the patient?
Approximately 15 to 60 minutes prior to each administration of Sarclisa, patients receive several medications to help reduce the chance of a reaction to the infusion:
- An H1 blocker antihistamine (e.g., diphenhydramine)
- An H2 blocker antihistamine (e.g., ranitidine)
- A painkiller and fever reducer (e.g., paracetamol)
- A corticosteroid (e.g., dexamethasone)
Sarclisa is administered via a tube into a vein (intravenous infusion, or i.v.) several times during a 28-day “treatment cycle”. During the first treatment cycle, Sarclisa is administered weekly on days 1, 8, 15, and 22. During the subsequent cycles, Sarclisa is administered every two weeks, on days 1 and 15. Sarclisa is administered in combination with pomalidomide (Pomalyst) and dexamethasone or in combination with carfilzomib and dexamethasone. Pomalidomide is taken by mouth once daily from day 1 to day 21 of each 28-day treatment cycle. Carfilzomib is administered by injection in a vein on days 1, 2, 8, 9, 15, and 16 of each 28-day treatment cycle. Low-dose dexamethasone (in addition to the corticosteroid that is injected into a vein before Sarclisa infusion) is given on days 1, 8, 15, and 22 for each 28-day cycle (either by mouth or by injection into a vein). On the days when both Sarclisa and carfilzomib are administered, dexamethasone is given first, followed by the Sarclisa, and then the carfilzomib infusion.

Patients may also receive antiviral or antibiotic medication to prevent herpes zoster (shingles) reactivation or infections during treatment with Sarclisa.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Multiple myeloma
In a clinical trial, 307 patients with multiple myeloma who had received prior treatment for their disease, and the treatment either did not work or stopped working, were treated with either: Sarclisa, pomalidomide (Pomalyst), and low-dose dexamethasone, OR pomalidomide and low-dose dexamethasone
At a median follow-up of 12 months:

(For the definition of “median”, click HERE.)
In another clinical trial, 302 patients with multiple myeloma who had received at least one prior treatment for their disease, and the treatment either did not work or stopped working, were treated with either:
- Sarclisa, carfilzomib, and dexamethasone OR
- carfilzomib and dexamethasone
At a median follow-up of 21 months:
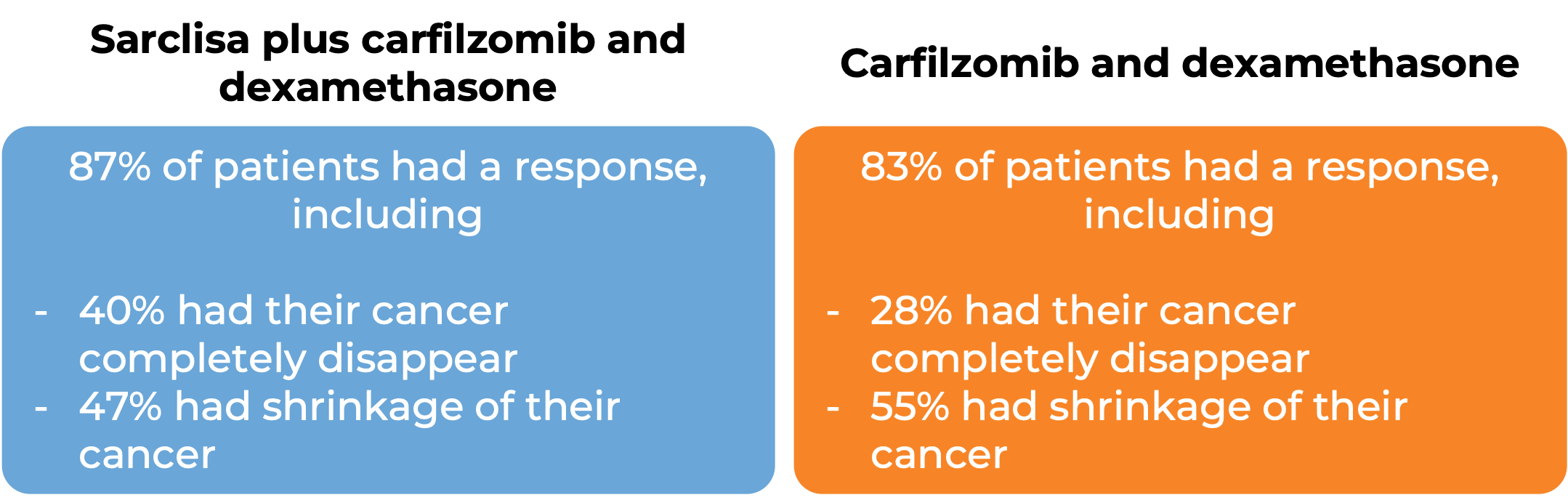
At a median follow-up of 44 months:
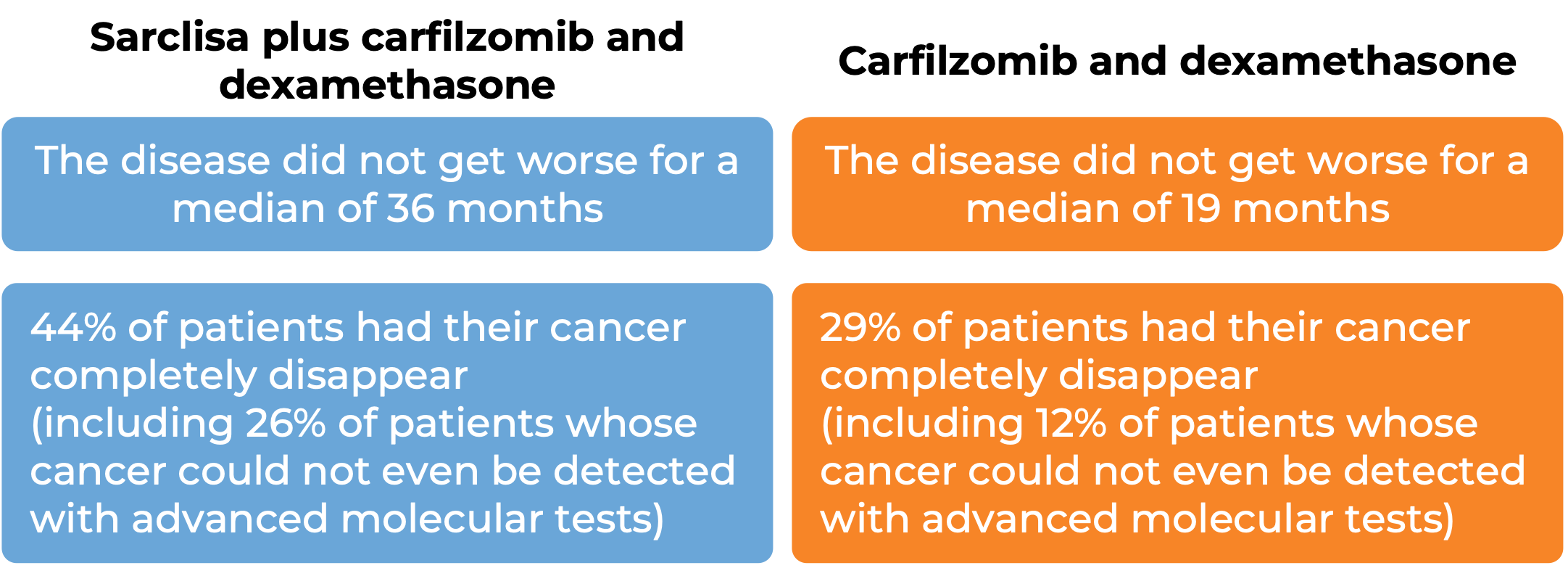
What are the side effects?
The most common side effects of Sarclisa include reactions related to the infusion (which can become serious such as cough, chills, nausea, difficulty breathing, and high blood pressure), low white blood cell count (which can lead to infections), lung infection (pneumonia), upper respiratory tract infection, difficulty breathing, diarrhea, nausea, vomiting, low red blood cell count, and low platelet count. Sarclisa may interfere with the results of blood tests that are needed before a patient can receive a blood transfusion. Tests to match a patient’s blood type should be done before the first dose of Sarclisa. In rare cases, Sarclisa can increase the risk of developing new cancers (including skin cancer). Cases of tumor lysis syndrome caused by the rapid breakdown of cancer cells, which can be severe or life-threatening, have also been reported.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Sanofi Aventis
Approval
FDA and EMA
Links to drug websites
Last updated on March 20, 2024








