How is this drug name pronounced?
Inotuzumab ozogamicin: ih-noh-TOO-zoo-mab OH-zoh-ga-MIH-sin
Besponsa: beh-SPON-suh
What cancer(s) does this drug treat?
Besponsa is approved for:
Advanced B cell precursor acute lymphoblastic leukemia (ALL)
- Patients with B cell precursor ALL that tests positive for the CD22 molecule who have received prior treatment for their disease, and the disease came back (relapsed disease), or the treatment either did not work, or stopped working (refractory disease).
Limitations of use:
Age: The safety and efficacy of Besponsa in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Besponsa may impair fertility in women and in men. In pregnant women, Besponsa may cause harm to the fetus and is not recommended for use during pregnancy. Women should not start Besponsa treatment when pregnant and are advised to use contraception during treatment with Besponsa and for at least 8 months after the last dose of Besponsa. Men are advised to use contraception during treatment with Besponsa and for at least 5 months after the last dose of Besponsa. The risks associated with Besponsa during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Besponsa and for at least 2 months after the last dose of Besponsa.
What type of immunotherapy is this?
How does this drug work?
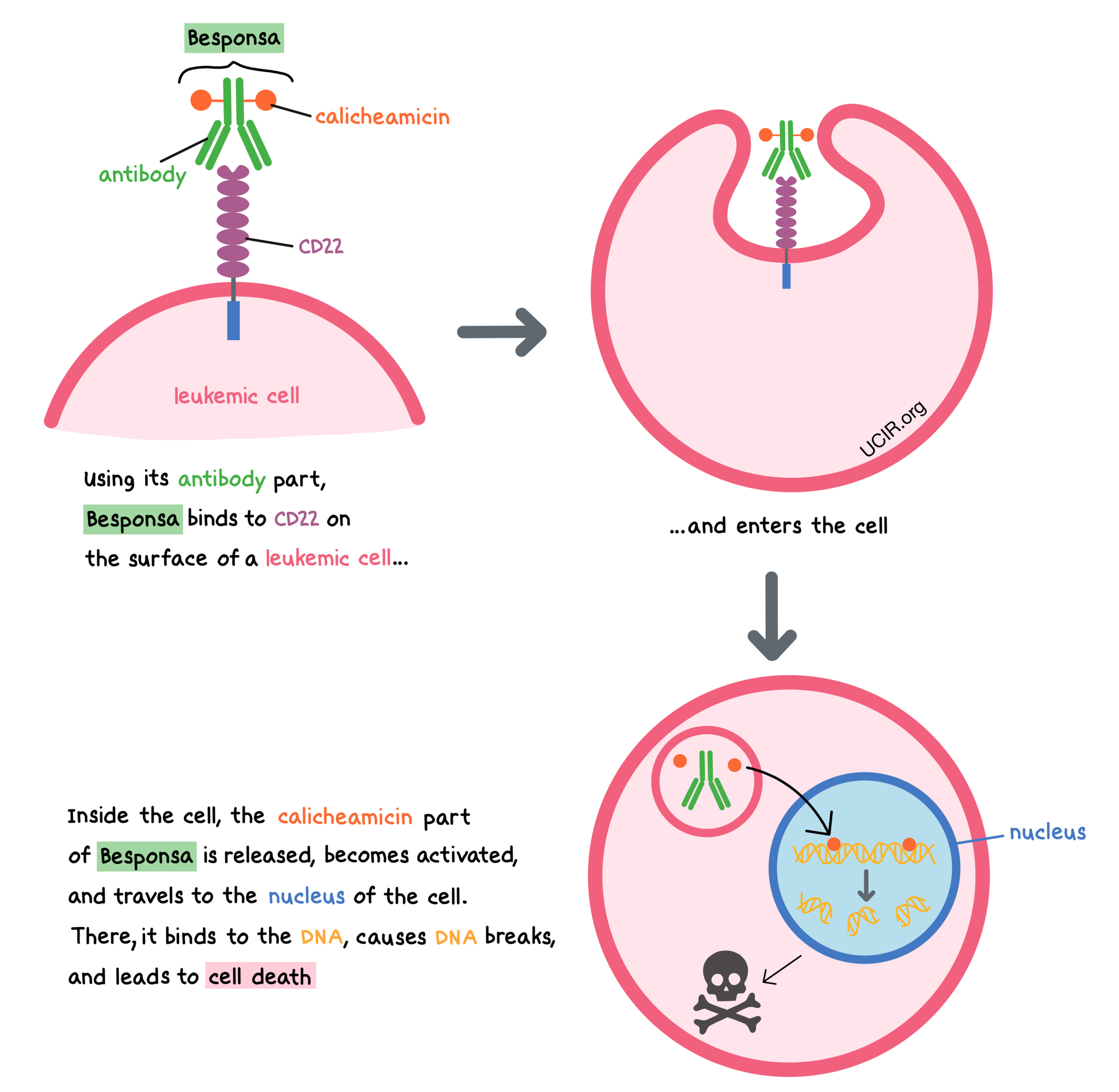
Besponsa is a medicine that consists of two parts that are connected: an antibody that attaches to a molecule called CD22 on the surface of acute lymphoblastic leukemia (ALL) cells, and a drug called calicheamicin that can kill cancer cells.
CD22 is present on the surface of some B cells, and in a healthy person, it regulates the immune response, preventing B cells from getting out of control and guarding against the development of autoimmune diseases. In B cell precursor ALL, the leukemic cells have CD22 on their surface. By targeting CD22, Besponsa is designed to minimize harm to normal, healthy cells and to bring the cell-killing drug directly to the leukemic cells. When Besponsa attaches to CD22 and enters the leukemic cell, the cell-killing drug part of Besponsa, calicheamicin, is released, becomes activated, and travels to the nucleus of the cell. There, it binds to the leukemic cell’s DNA, causes DNA breaks, and leads to cell death.
How is this drug given to the patient?
Prior to each administration of Besponsa, patients receive several medications to help reduce the chance of a reaction to the infusion:
- A corticosteroid
- An antihistamine
- A fever reducer (e.g., acetaminophen)
Patients are observed for at least one hour after administration of Besponsa for any symptoms related to the infusion.
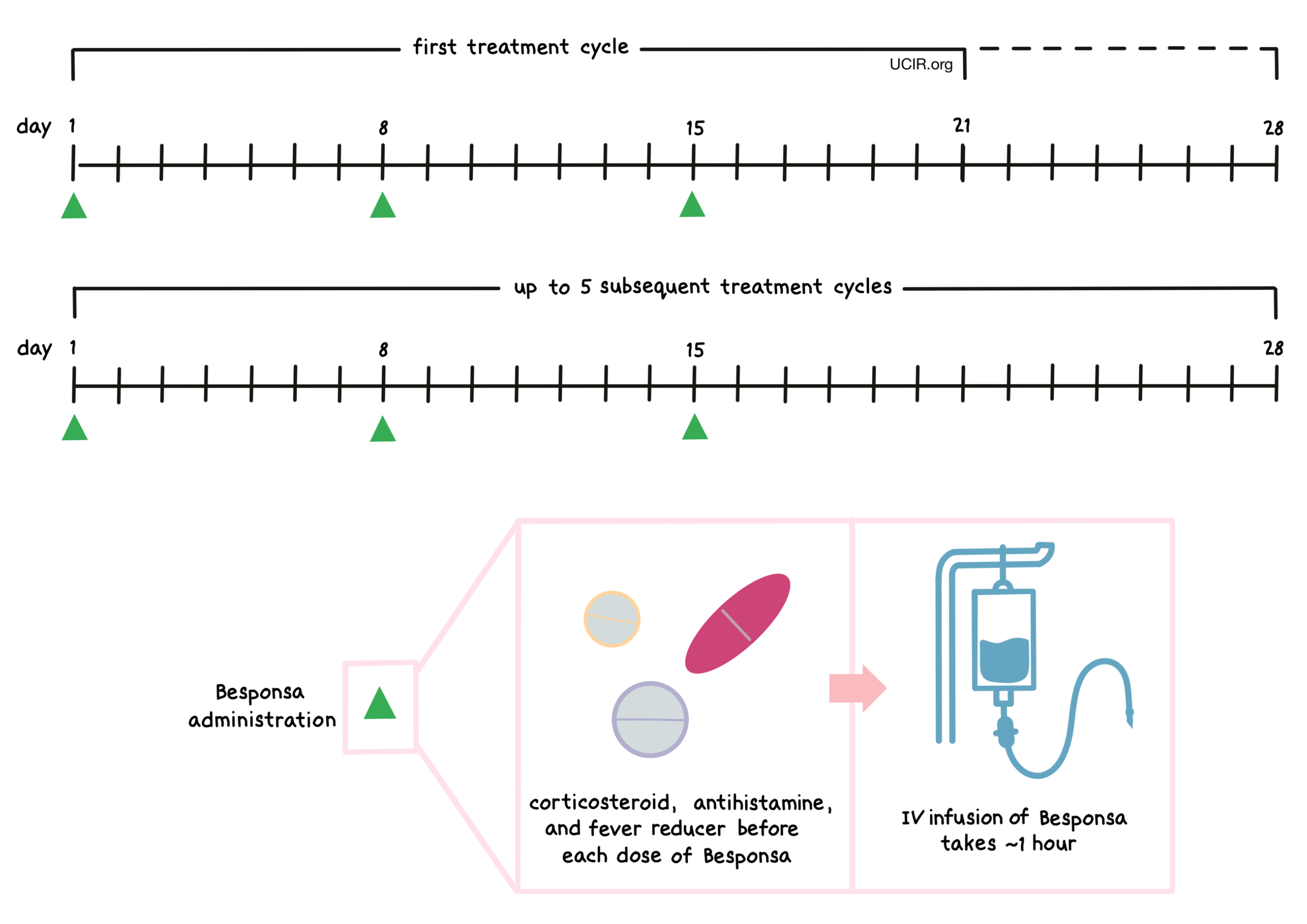
Besponsa is administered via a tube into a vein (intravenous infusion, or I.V.) over one hour on days 1, 8, and 15, followed by 6 or 13 days without treatment. The first “treatment cycle” is 21 days long, but may be extended to 28 days; all subsequent cycles are 28 days long. If the patient is going to receive a stem cell transplant, then the patient receives only 2 or 3 treatment cycles of Besponsa; otherwise, the patient will receive a maximum of 6 treatment cycles of Besponsa. If the patient does not achieve remission by the third cycle, treatment with Besponsa should be stopped. Infusion of Besponsa does not require a hospital stay.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies.
Advanced B cell precursor acute lymphoblastic leukemia (ALL)
In a clinical trial, 326 patients with B cell precursor ALL that tested positive for the CD22 molecule, who had received one or two previous chemotherapy treatments for their disease (and at least one tyrosine kinase inhibitor treatment for patients whose ALL tested positive for the Philadelphia chromosome), and the disease came back, or the treatment either did not work, or stopped working, were treated with either Besponsa or investigator’s choice of chemotherapy (FLAG [Fludarabine + cytarabine + granulocyte colony-stimulating factor], MXN/ARaC [mitoxantrone + cytarabine], or HIDAC [High-dose cytarabine]).
Among the first 218 treated patients:
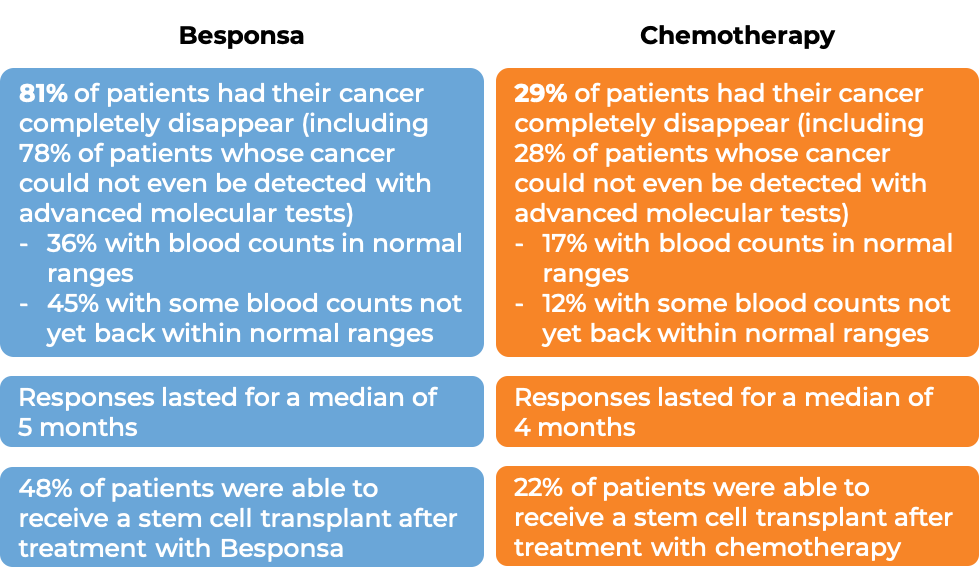
(For the definition of “median”, click HERE.)
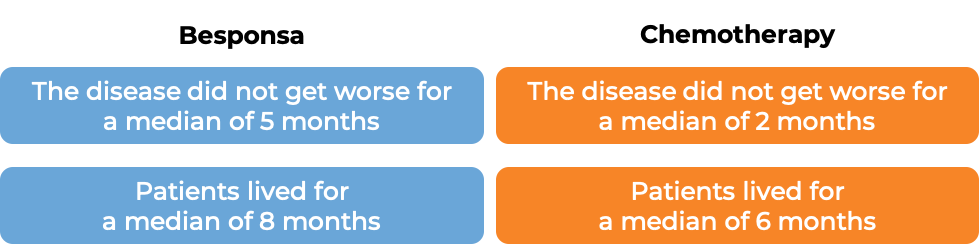
The clinical results observed for the first 218 treated patients were consistent with those seen in all 326 patients.
What are the side effects?
The most common side effects of Besponsa include infections, fatigue, bleeding, fever, nausea, headache, pain in the abdomen, low platelet count, low white blood cell count (with or without fever), low red blood cell count, and abnormal liver function.
Besponsa can cause side effects that can become serious or life-threatening and may lead to death. Some of the serious side effects related to Besponsa include problems with the liver or the heart, low blood cell counts and the associated complications (including severe bleeding and infections), and increased risk of death after stem cell transplant (due to infections or liver problems). Reactions related to the infusion may also occur. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
Severe liver problems
Besponsa may cause severe, life-threatening, or fatal liver problems, including a condition called veno-occlusive disease (VOD). VOD occurs when small blood vessels that lead into the liver or are inside the liver become blocked. VOD can occur during treatment with Besponsa or during a subsequent stem cell transplant. Symptoms of VOD may include rapid weight gain, abdominal swelling that may be painful, and yellowing of the whites of the eyes.
Patients may be at a higher risk of VOD if they:
- Have elevated lab test indicators that measure abnormal liver function
- Have received two alkylating agents in preparation for their stem cell transplant
- Receive a stem cell transplant after being treated with Besponsa
- Have previously received a stem cell transplant
- Have a history of liver problems or ongoing liver problems
- Are of an older age
- Have received multiple treatments for their disease prior to receiving Besponsa
- Receive a greater number of Besponsa treatment cycles
Treatment with Besponsa may be modified based on periodic liver test results, and should be permanently discontinued if VOD occurs.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Pfizer
Approval
FDA and EMA
Links to drug websites
Last updated on January 5, 2021
How is this drug name pronounced?
Inotuzumab ozogamicin: ih-noh-TOO-zoo-mab OH-zoh-ga-MIH-sin
Besponsa: beh-SPON-suh
What cancer(s) does this drug treat?
Besponsa is approved for:
Advanced B cell precursor acute lymphoblastic leukemia (ALL)
- Patients with B cell precursor ALL that tests positive for the CD22 molecule who have received prior treatment for their disease, and the disease came back (relapsed disease), or the treatment either did not work, or stopped working (refractory disease). Patients whose disease tests positive for the Philadelphia chromosome have to have tried at least one treatment with a tyrosine kinase inhibitor prior to receiving Besponsa.
Limitations of use:
Age: The safety and efficacy of Besponsa in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Besponsa may impair fertility in women and in men. In pregnant women, Besponsa may cause harm to the fetus and is not recommended for use during pregnancy. Women should not start Besponsa treatment when pregnant and are advised to use contraception during treatment with Besponsa and for at least 8 months after the last dose of Besponsa. Men are advised to use contraception during treatment with Besponsa and for at least 5 months after the last dose of Besponsa. The risks associated with Besponsa during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Besponsa and for at least 2 months after the last dose of Besponsa.
Other: Besponsa should not be administered to patients who have a history of or have ongoing veno-occlusive liver disease/sinusoidal obstruction syndrome (VOD/SOS). Besponsa should not be administered to patients who have serious ongoing liver disease.
Vaccinations: Live virus vaccinations (e.g., chickenpox or measles, mumps, and rubella (MMR)) should not be given within the 2 weeks prior to Besponsa treatment, during Besponsa treatment, or before B cells recover to normal levels after the last treatment with Besponsa. recovery of normal B cell levels after the last treatment.
What type of immunotherapy is this?
How does this drug work?
Besponsa is a medicine that consists of two parts that are connected: an antibody that attaches to a molecule called CD22 on the surface of acute lymphoblastic leukemia (ALL) cells, and a drug called calicheamicin that can kill cancer cells.
CD22 is present on the surface of some B cells, and in a healthy person, it regulates the immune response, preventing B cells from getting out of control and guarding against the development of autoimmune diseases. In B cell precursor ALL, the leukemic cells have CD22 on their surface. By targeting CD22, Besponsa is designed to minimize harm to normal, healthy cells and to bring the cell-killing drug directly to the leukemic cells. When Besponsa attaches to CD22 and enters the leukemic cell, the cell-killing drug part of Besponsa, calicheamicin, is released, becomes activated, and travels to the nucleus of the cell. There it binds to the leukemic cell’s DNA, causes DNA breaks, and leads to cell death.

How is this drug given to the patient?
Prior to each administration of Besponsa, patients receive several medications to help reduce the chance of a reaction to the infusion:
- A corticosteroid
- An antihistamine
- A fever reducer (e.g., paracetamol)
Besponsa is administered via a tube into a vein (intravenous infusion, or I.V.) over one hour on days 1, 8, and 15. Patients are observed for at least one hour after administration of Besponsa for any symptoms related to the infusion. The first “treatment cycle” is 21 days long, but may be extended to 28 days; all subsequent treatment cycles are 28 days long. If the patient is going to receive a stem cell transplant, the patient will receive only 2 or 3 treatment cycles of Besponsa; otherwise, the patient will receive a maximum of 6 treatment cycles of Besponsa. If the patient does not achieve remission by the third cycle, treatment with Besponsa should be stopped. Infusion of Besponsa does not require a hospital stay.

Patients with a high amount of cancer in their body may receive additional treatment (“prophylaxis”) prior to receiving Besponsa to prevent tumor lysis syndrome, a serious side effect caused by the rapid breakdown of cancerous cells. Prophylaxis consists of adequate hydration and administration of drugs that help prevent the buildup of uric acid in the blood.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies.
Advanced B cell precursor acute lymphoblastic leukemia (ALL)
In a clinical trial, 326 patients with B cell precursor ALL that tested positive for the CD22 molecule, who had received one or two previous chemotherapy treatments for their disease (and at least one tyrosine kinase inhibitor treatment for patients whose ALL tested positive for the Philadelphia chromosome), and the disease came back, or the treatment either did not work, or stopped working, were treated with either Besponsa or investigator’s choice of chemotherapy (FLAG [Fludarabine + cytarabine + granulocyte colony-stimulating factor], MXN/ARaC [mitoxantrone + cytarabine], or HIDAC [High-dose cytarabine]).
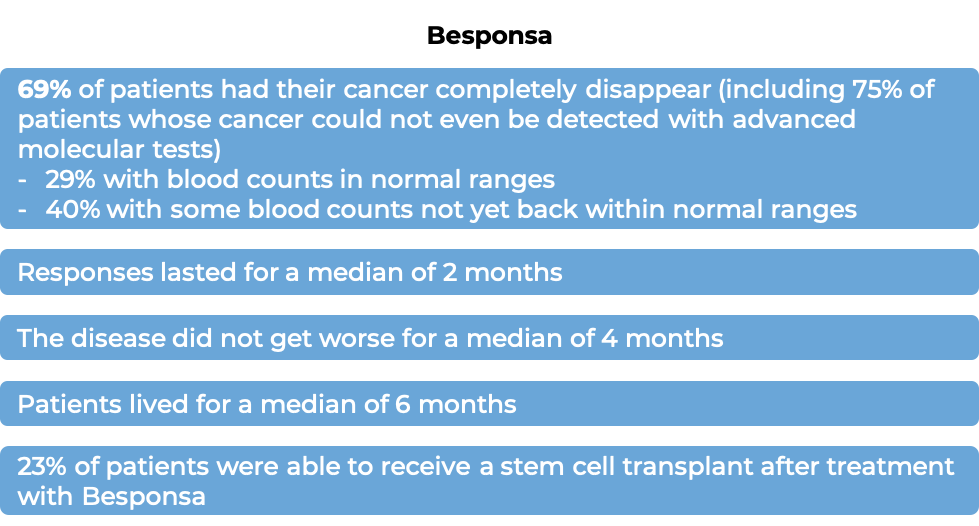
Among the first 218 treated patients:

(For the definition of “median”, click HERE.)
The clinical results observed for the first 218 treated patients were consistent with those seen in all 326 patients. Among all 326 treated patients:
In another clinical trial, 35 patients with B cell precursor ALL that tested positive for the CD22 molecule, who had received at least two previous treatments for their disease, and the disease came back, or the treatment either did not work, or stopped working, were treated with Besponsa.
What are the side effects?
The most common side effects of Besponsa include infections, fatigue, bleeding, fever, nausea, headache, pain in the abdomen, low platelet count, low white blood cell count (with or without fever), low red blood cell count, and abnormal liver function.
Besponsa can cause side effects that can become serious or life-threatening and may lead to death. Some of the serious side effects related to Besponsa include problems with the liver or the heart, low blood cell counts and the associated complications (including severe bleeding and infections), increased risk of death after stem cell transplant (due to infections or liver problems), and tumor lysis syndrome. Reactions related to the infusion may also occur. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
Severe liver problems
Besponsa may cause severe, life-threatening, or fatal liver problems, including a condition called veno-occlusive liver disease/sinusoidal obstruction syndrome (VOD/SOS). VOD/SOS occurs when small blood vessels that lead into the liver or are inside the liver become blocked. VOD/SOS can occur during treatment with Besponsa or during a subsequent stem cell transplant. Symptoms of VOD/SOS may include rapid weight gain, abdominal swelling that may be painful, abnormal buildup of fluid in the abdomen, and yellowing of the whites of the eyes.
Patients may be at a higher risk of VOD/SOS after being treated with Besponsa and subsequent stem cell transplant if they:
- Have elevated lab test indicators that measure abnormal liver function
- Have received two alkylating agents in preparation for their stem cell transplant
- Have previously received a stem cell transplant
- Have a history of liver problems or ongoing liver problems
- Are 65 years old or older
- Have received multiple treatments for their disease prior to receiving Besponsa
- Receive a greater number of Besponsa treatment cycles
Treatment with Besponsa may be modified based on periodic liver test results, and should be permanently discontinued if VOD/SOS occurs.
Tumor Lysis Syndrome (TLS)
Tumor lysis syndrome is caused by the rapid breakdown of cancer cells. TLS can cause kidney failure. Symptoms of TLS include nausea, vomiting, diarrhea, and lack of energy.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Pfizer
Approval
FDA and EMA
Links to drug websites
Last updated on January 13, 2023







