How is the drug name pronounced?
Ibritumomab: EYE-brih-TOO-moe-MAB
Zevalin: ZEV-ah-lin
What cancer(s) does this drug treat?
Non-Hodgkin lymphoma (NHL)
Zevalin in combination with rituximab (Rituxan or Truxima) is approved for:
- Patients with follicular Non-Hodgkin lymphoma whose cancer has shrunk or disappeared after their first treatment with chemotherapy. In such cases, Zevalin is given to further improve the response to treatment (consolidation therapy).
- Patients with low-grade or follicular Non-Hodgkin lymphoma who have previously received treatment for their disease, and the disease came back (relapsed disease), or the treatment either did not work or stopped working (refractory disease).
Limitations of Use
Age: The safety and efficacy of Zevalin have not been established in patients under 18 years of age.
Vaccinations: Live virus vaccinations (e.g., chickenpox or measles, mumps, and rubella (MMR)) prior to or during Zevalin treatment are not recommended.
Fertility/Pregnancy/breastfeeding: Zevalin may impair fertility in women and in men. In pregnant women, Zevalin may cause harm to the fetus and is not recommended for use during pregnancy. Women and men are advised to use contraception during treatment with Zevalin and for at least 12 months after treatment with Zevalin. The risks associated with Zevalin during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Zevalin and for at least 6 months after the last dose of Zevalin.
What type of immunotherapy is this?
How does this drug work?
Target:
Zevalin is a man-made antibody that delivers radioactive material (Yttrium-90) directly to cancer cells. Zevalin and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets.
Zevalin is usually given in combination with another therapeutic antibody, rituximab (Rituxan or Truxima). Zevalin and rituximab are different antibodies, but both bind to a molecule called CD20 on the surface of a cancerous B cell. CD20 is commonly found on the surface of cancer cells in patients with Non-Hodgkin lymphoma. CD20 can also be found on the surface of all normal human B cells, which means that in addition to attacking cancer cells, Zevalin and rituximab can also attack healthy B cells.
Rituximab is given to reduce the amount of B cells in the blood before the administration of Zevalin. When bound to CD20 on the surface of cancerous or normal B cells, the radioactive molecule Yttrium-90, which is attached to Zevalin, will decay and emit (release) short wavelength beta radiation. This radiation damages and kills the cells Zevalin is bound to (along with neighboring cells that are in very close proximity) while limiting the damage to surrounding normal tissue. Zevalin may also activate an immune response against the cancer cells.
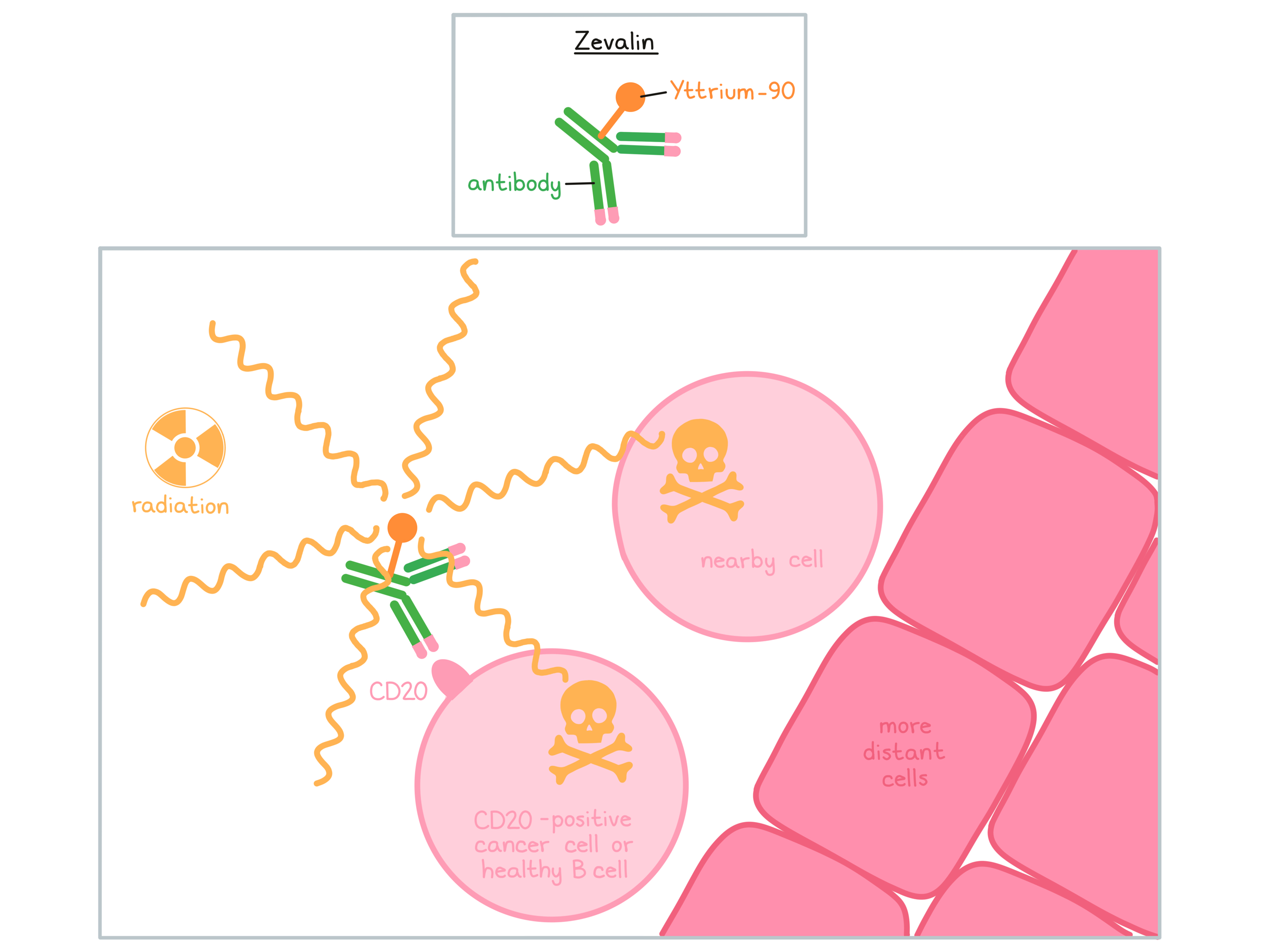
The combined effect of rituximab and Zevalin results in the elimination of cancerous and normal B cells from the body. A new population of healthy B cells can then develop from blood-forming “stem cells” that reside in the bone marrow.
How is this drug given to the patient?
Zevalin is administered in combination with rituximab (Rituxan or Truxima). Administration of rituximab and Zevalin does not require a hospital stay, but should be administered in a specialized facility.
As part of treatment with Zevalin, patients receive treatment with rituximab. Before each infusion of rituximab, acetaminophen (e.g., Tylenol) and an antihistamine (e.g., Benadryl) are given to help reduce the chance of a reaction to the infusion. Rituximab is administered via a tube into a vein (intravenous infusion, or I.V.) over several hours on day 1 and on day 7, 8, or 9. The first infusion of rituximab is initially administered slowly in order to monitor for unwanted reactions to the infusion. If any infusion reactions occur, the dose may be lowered, slowed, temporarily withheld, or discontinued at the doctor’s discretion. In the absence of any such reactions, the dosage rate is increased until the full dose has been administered.
Zevalin is administered within 4 hours of the second rituximab infusion on day 7, 8, or 9 via infusion into a vein. The infusion takes about 10 minutes.
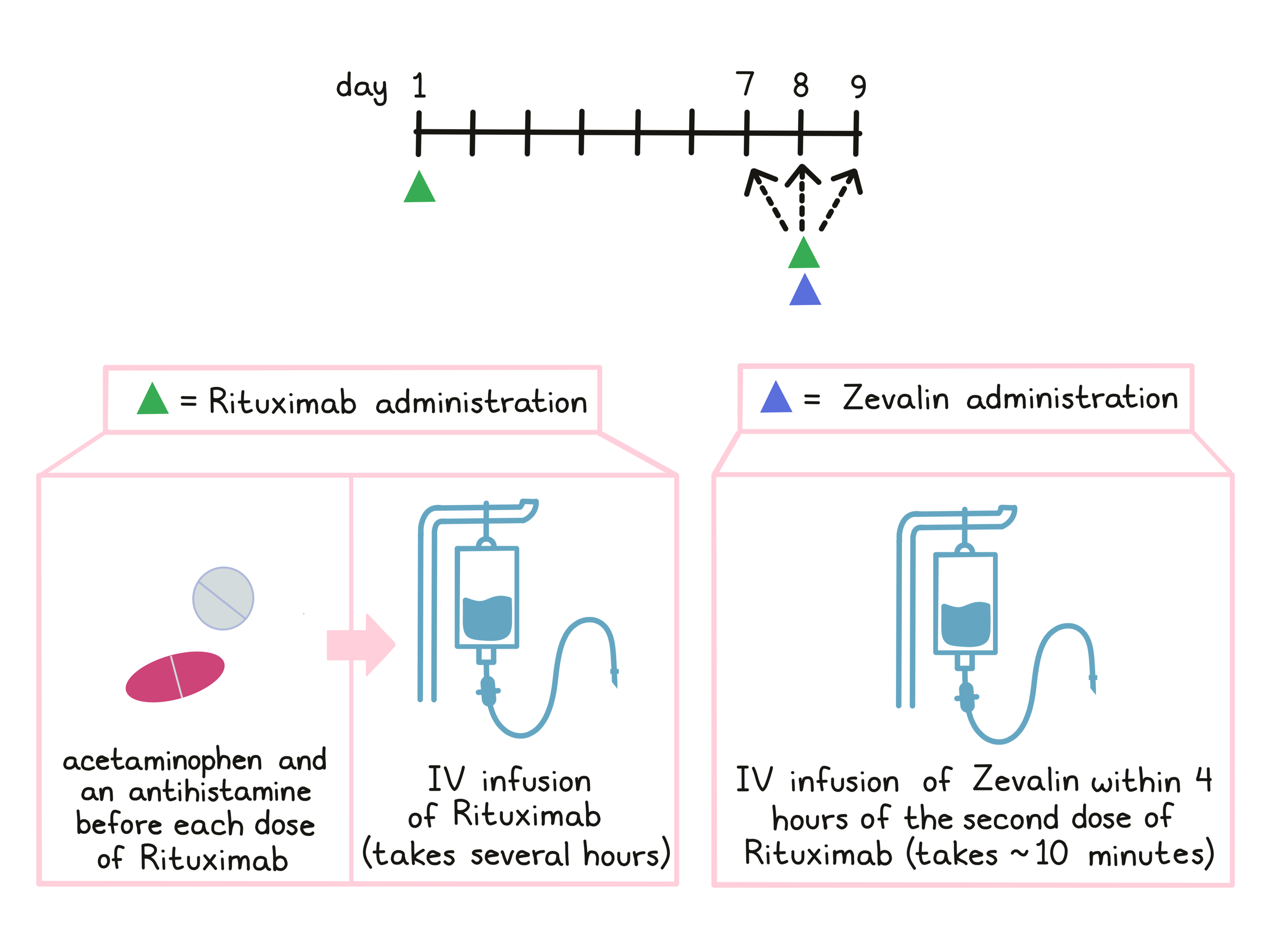
For patients who are treated with Zevalin as consolidation therapy, following their first treatment with chemotherapy, Zevalin treatment is initiated after the recovery of the patient’s platelet counts at least 6 weeks, but no more than 12 weeks after the final dose of chemotherapy.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
In a clinical trial, 414 patients with follicular Non-Hodgkin lymphoma whose cancer had shrunk or disappeared in response to first treatment with chemotherapy, were either treated with Zevalin, or put on observation with no further treatment. Patients treated with Zevalin lived for a median of 38 months without experiencing a worsening of their disease, compared to a median of 18 months for patients receiving no further treatment.
Low-grade or Follicular Lymphoma (previously treated)
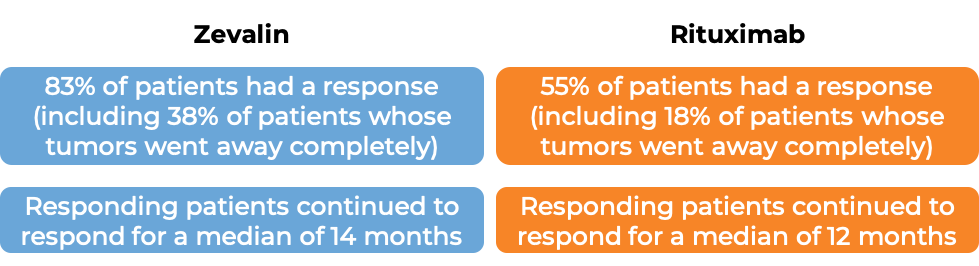
In a clinical trial, 54 patients with follicular lymphoma who had previously received treatment for their disease, and the disease came back and did not respond to treatment with rituximab (Rituxan or Truxima), were treated with Zevalin. 74% of patients had their tumors shrink, including 15% whose tumors disappeared entirely. For patients who saw their tumors get smaller or disappear, the disease did not get worse for a median of 6 months.
In another clinical trial, 130 patients with low-grade or follicular Non-Hodgkin's lymphoma who have previously received treatment (excluding rituximab) for their disease, and the disease did not respond to treatment or came back, were either treated with Zevalin or with rituximab (Rituxan or Truxima).
What are the potential side effects?
Zevalin and rituximab (Rituxan or Truxima) target the CD20 molecule which, while present on cancerous B cells, is also present on normal B cells. As a result, treatment can kill normal B cells, increasing the risk of infections including bronchitis, common cold, and urinary tract infections. Other common side effects of Zevalin include fatigue, weakness, fever, abdominal pain, nausea, diarrhea, dizziness, coughing, rashes, night sweats, high blood pressure, low white or red blood cell counts (including low numbers of neutrophils and/or lymphocytes), and low blood platelet counts. Zevalin treatment can increase the risk of developing another cancer such as myelodysplastic syndrome, or acute myelogenous leukemia.
Some side effects, such as infusion-related reactions, extended and severe cytopenias, and severe skin reactions, can become life-threatening, and may lead to death. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
Infusion-related reactions
Reactions to the rituximab infusion as part of treatment with Zevalin are common and usually begin 30 to 120 minutes after the start of the infusion. Symptoms include hives, rashes, swelling of the lips, tongue, or face, sudden cough, shortness of breath, difficulty breathing, dizziness or feeling faint, heart racing or fluttering, and chest pain. If symptoms of an infusion-related reaction occur at any point during the first infusion or any subsequent infusions, the infusion may be interrupted or slowed, depending on the severity of the reaction. It is important that patients discuss with their doctor if they notice any changes during or following a rituximab infusion, as infusion-related reactions are very serious and can lead to death.
Another potential side effect of Zevalin treatment is leakage of Zevalin or rituximab from the vein it was administered to into the surrounding tissue. Patients should immediately report burning, pain, stinging, redness or swelling around the injection or infusion site to their healthcare provider.
Extended and severe decreases in blood cell counts
Decreases in blood cell counts (cytopenias) are common with Zevalin treatment and can continue for more than 12 weeks after receiving Zevalin. Symptoms include fatigue, weakness, shortness of breath, difficulty concentrating, dizziness, light-headedness, cold hands or feet, bruises, red or purple spots on the skin, unusual bleeding, and increased risk of infection.
Severe skin or mucous membrane reactions
Zevalin treatment may cause serious and sometimes life-threatening skin or mucous membrane reactions that may lead to death. Symptoms include fever, reddening of the skin, itchy skin, rashes, blisters, mouth sores, and flaking of the skin.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
US: Acrotech Biopharma LLC
Europe: Ceft Biopharma
Approval
FDA and EMA
Links to drug websites
Last updated on January 12, 2021
How is the drug name pronounced?
Ibritumomab: EYE-brih-TOO-moe-MAB
Zevalin: ZEV-ah-lin
What cancer(s) does this drug treat?
Non-Hodgkin lymphoma (NHL)
Zevalin in combination with rituximab (e.g., MabThera) is approved for:
- Patients with follicular Non-Hodgkin lymphoma whose cancer has shrunk or disappeared after their first treatment with chemotherapy. In such cases, Zevalin is given to further improve the response to treatment (consolidation therapy).
- Patients with follicular Non-Hodgkin lymphoma who have previously received treatment for their disease, and the disease came back (relapsed disease), or the treatment either did not work or stopped working (refractory disease).
Limitations of Use
Age: The safety and efficacy of Zevalin have not been established in patients under 18 years of age.
Exclusions: Zevalin is not recommended for use in patients with follicular Non-Hodgkin lymphoma that has formed in the brain or spread to the brain.
Fertility/Pregnancy/breastfeeding: Zevalin may impair fertility in women and in men. In pregnant women, Zevalin may cause harm to the fetus and is not recommended for use during pregnancy. Women should not start Zevalin treatment when pregnant. Women are advised to use contraception during treatment with Zevalin and for at least 12 months after treatment with Zevalin. The risks associated with Zevalin during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Zevalin and for up to 12 months after Zevalin treatment.
Vaccinations: Live virus vaccinations (e.g., chickenpox or measles, mumps, and rubella (MMR)) prior to or during Zevalin treatment are not recommended.
Treatment with growth factors: Patients should not receive treatment with growth factors that can increase the production of blood cells 3 weeks before and 2 weeks after treatment with Zevalin.
Human anti-murine antibodies: Patients who have been previously treated with therapeutic antibodies that were made in a laboratory and were developed from an antibody that was isolated from a mouse must be tested for the presence of antibodies in their blood that can bind to mouse antibodies prior to treatment with Zevalin. The presence of these antibodies has the potential to cause hypersensitivity or allergic reactions to the Zevalin.
What type of immunotherapy is this?
How does this drug work?
Target:
Zevalin is a man-made antibody that delivers radioactive material (Yttrium-90) directly to cancer cells. Zevalin and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets.
Zevalin is usually given in combination with another therapeutic antibody, rituximab (e.g., MabThera). Zevalin and rituximab are different antibodies, but both bind to a molecule called CD20 on the surface of a cancerous B cell. CD20 is commonly found on the surface of cancer cells in patients with Non-Hodgkin lymphoma. CD20 can also be found on the surface of all normal human B cells, which means that in addition to attacking cancer cells, Zevalin and rituximab can also attack healthy B cells.
Rituximab is given to reduce the amount of B cells in the blood before the administration of Zevalin. When bound to CD20 on the surface of cancerous or normal B cells, the radioactive molecule Yttrium-90, which is attached to Zevalin, will decay and emit (release) short wavelength beta radiation. This radiation damages and kills the cells Zevalin is bound to (and neighboring cells that are in very close proximity) while limiting the damage to surrounding normal tissue. Zevalin may also activate an immune response against the cancer cells.

The combined effect of rituximab and Zevalin results in the elimination of cancerous and normal B cells from the body. A new population of healthy B cells can then develop from blood-forming “stem cells” that reside in the bone marrow.
How is this drug given to the patient?
Zevalin is administered in combination with rituximab (e.g., MabThera). Administration of rituximab and Zevalin does not require a hospital stay, but should be administered in a specialized facility.
As part of treatment with Zevalin, patients receive treatment with rituximab. Before each infusion of rituximab, paracetamol and an antihistamine are given to help reduce the chance of a reaction to the infusion. Rituximab is administered via a tube into a vein (intravenous infusion, or I.V.) over several hours on day 1 and on day 7, 8, or 9. The first infusion of rituximab is initially administered slowly in order to monitor for unwanted reactions to the infusion. If any infusion reactions occur, the dose may be lowered, slowed, temporarily withheld, or discontinued at the doctor’s discretion. In the absence of any such reactions, the dosage rate is increased until the full dose has been administered. The second rituximab infusion on day 7, 8, or 9 should be completed within 4 hours.
Zevalin is administered after the second rituximab dose on day 7, 8, or 9 via infusion into a vein. The Zevalin infusion takes about 10 minutes.
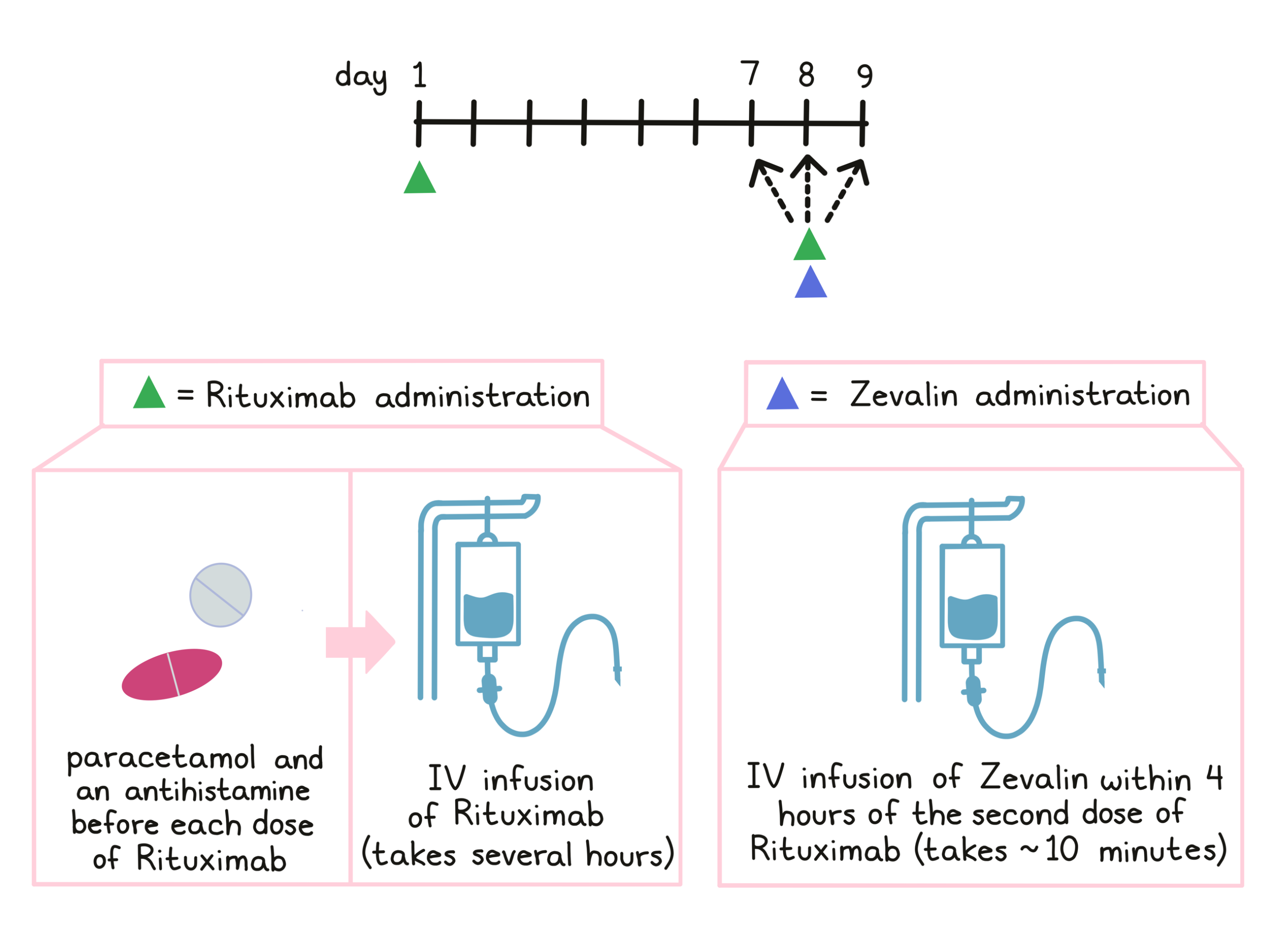
For patients who are treated with Zevalin as consolidation therapy following their first treatment with chemotherapy, Zevalin treatment is initiated after the recovery of the patient’s platelet counts at least 6 weeks, but no more than 12 weeks after the final dose of chemotherapy.
In most patients, Zevalin treatment results in an extended and severe decrease in blood cell counts. After Zevalin treatment, patients will be monitored, and on a weekly basis, they need to get blood work done until blood cell counts are back to a normal range.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Follicular Non-Hodgkin lymphoma (consolidation therapy)
In a clinical trial, 414 patients with follicular Non-Hodgkin lymphoma whose cancer had shrunk or disappeared in response to first treatment with chemotherapy, were either treated with Zevalin, or put on observation with no further treatment. At a median follow-up of 2.9 years, patients treated with Zevalin lived for a median of 37 months without experiencing a worsening of their disease, compared to a median of 14 months for patients receiving no further treatment.
Low-grade or Follicular Lymphoma (previously treated)
In a clinical trial, 54 patients with follicular lymphoma who had previously received treatment for their disease, and the disease came back and did not respond to treatment with rituximab (e.g., MabThera), were treated with Zevalin. 74% of patients had their tumors shrink, including 15% whose tumors disappeared entirely. For patients who saw their tumors get smaller or disappear, the disease did not get worse for a median of 6 months.
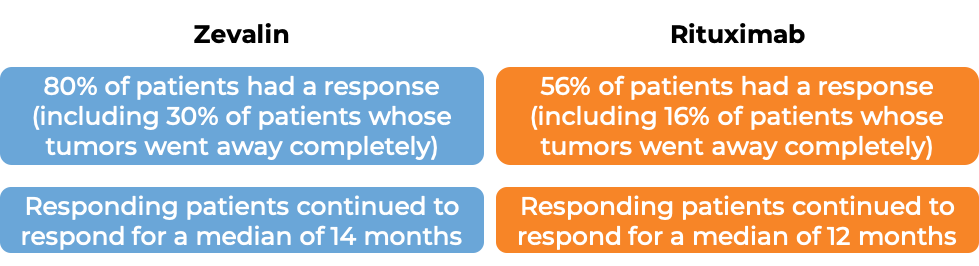
In another clinical trial, 130 patients with low-grade or follicular Non-Hodgkin's lymphoma who had previously received treatment for their disease (excluding rituximab), and the disease did not respond to treatment or came back, were either treated with Zevalin or with rituximab (e.g., MabThera).
What are the potential side effects?
Zevalin and rituximab (e.g., MabThera) target the CD20 molecule, which, while present on cancerous B cells, is also present on normal B cells. As a result, treatment can kill normal B cells, increasing the risk of infections, including pneumonia and urinary tract infections. Other common side effects of Zevalin include fatigue, weakness, fever, abdominal pain, nausea, diarrhea, dizziness, coughing, rashes, night sweats, high blood pressure, low white or red blood cell counts (including low numbers of neutrophils and/or lymphocytes), and low blood platelet counts. Zevalin treatment can increase the risk of developing another cancer, such as myelodysplastic syndrome, or acute myelogenous leukemia.
Some side effects, such as infusion-related reactions, extended and severe cytopenias, and severe skin reactions, can become life-threatening, and may lead to death. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
Infusion-related reactions
Reactions to the rituximab infusion as part of treatment with Zevalin are common and usually begin 30 to 120 minutes after the start of the infusion. Symptoms include hives, rashes, swelling of the lips, tongue, or face, sudden cough, shortness of breath, difficulty breathing, dizziness or feeling faint, heart racing or fluttering, and chest pain. If symptoms of an infusion-related reaction occur at any point during the first infusion or any subsequent infusions, the infusion may be interrupted or slowed, depending on the severity of the reaction. It is important that patients discuss with their doctor if they notice any changes during or following a rituximab infusion, as infusion-related reactions are very serious and can lead to death.
Another potential side effect of Zevalin treatment is leakage of Zevalin or rituximab from the vein it was administered into the surrounding tissue. Patients should immediately report burning, pain, stinging, redness or swelling around the injection or infusion site to their healthcare provider.
Extended and severe decreases in blood cell counts
Decreases in blood cell counts (cytopenias) are common with Zevalin treatment and can continue for more than 12 weeks after receiving Zevalin. Symptoms include fatigue, weakness, shortness of breath, difficulty concentrating, dizziness, light-headedness, cold hands or feet, bruises, red or purple spots on the skin, unusual bleeding, and increased risk of infection.
Severe skin or mucous membrane reactions
Zevalin treatment may cause serious and sometimes life-threatening skin or mucous membrane reactions that may lead to death. Symptoms include fever, reddening of the skin, itchy skin, rashes, blisters, mouth sores, and flaking of the skin.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
US: Acrotech Biopharma LLC
Europe: Ceft Biopharma
Approval
FDA and EMA
Links to drug websites
Last updated on January 12, 2021







