How is this drug name pronounced?
Gemtuzumab ozogamicin: gem-TOO-zoo-mab OH-zoh-ga-MIH-sin
Mylotarg: MY-loh-targ
What cancer(s) does this drug treat?
Acute myeloid leukemia (AML)
Mylotarg is approved for:
- Adult and pediatric patients 1 month and older with newly diagnosed acute myeloid leukemia that tests positive for the CD33 molecule. In such cases, Mylotarg may be used with or without chemotherapy.
- Adult and pediatric patients 2 years and older with acute myeloid leukemia that tests positive for the CD33 molecule, who have previously received treatment for their disease, and the disease came back (relapsed disease), or the treatment either did not work or stopped working (refractory disease).
Limitations of use:
Age: The safety and efficacy of Mylotarg in combination with chemotherapy in patients under 1 month of age who have newly diagnosed acute myeloid leukemia have not been established. The safety and efficacy of Mylotarg as a single agent (without chemotherapy) in patients under 18 years of age with newly diagnosed acute myeloid leukemia and in patients under 2 years of age with relapsed or refractory acute myeloid leukemia have not been established.
Fertility/Pregnancy/Breastfeeding: Mylotarg may impair fertility in women and in men. In pregnant women, Mylotarg may cause harm to the fetus and is not recommended for use during pregnancy. Women are advised to use contraception during treatment with Mylotarg and for at least 6 months after the last dose of Mylotarg. The risks associated with Mylotarg during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Mylotarg and for at least 1 month after the last dose of Mylotarg. Men are advised to use contraception during treatment with Mylotarg and for at least 3 months after the last dose of Mylotarg.
What type of immunotherapy is this?
- Antibody–drug conjugate
How does this drug work?
- Target: CD33
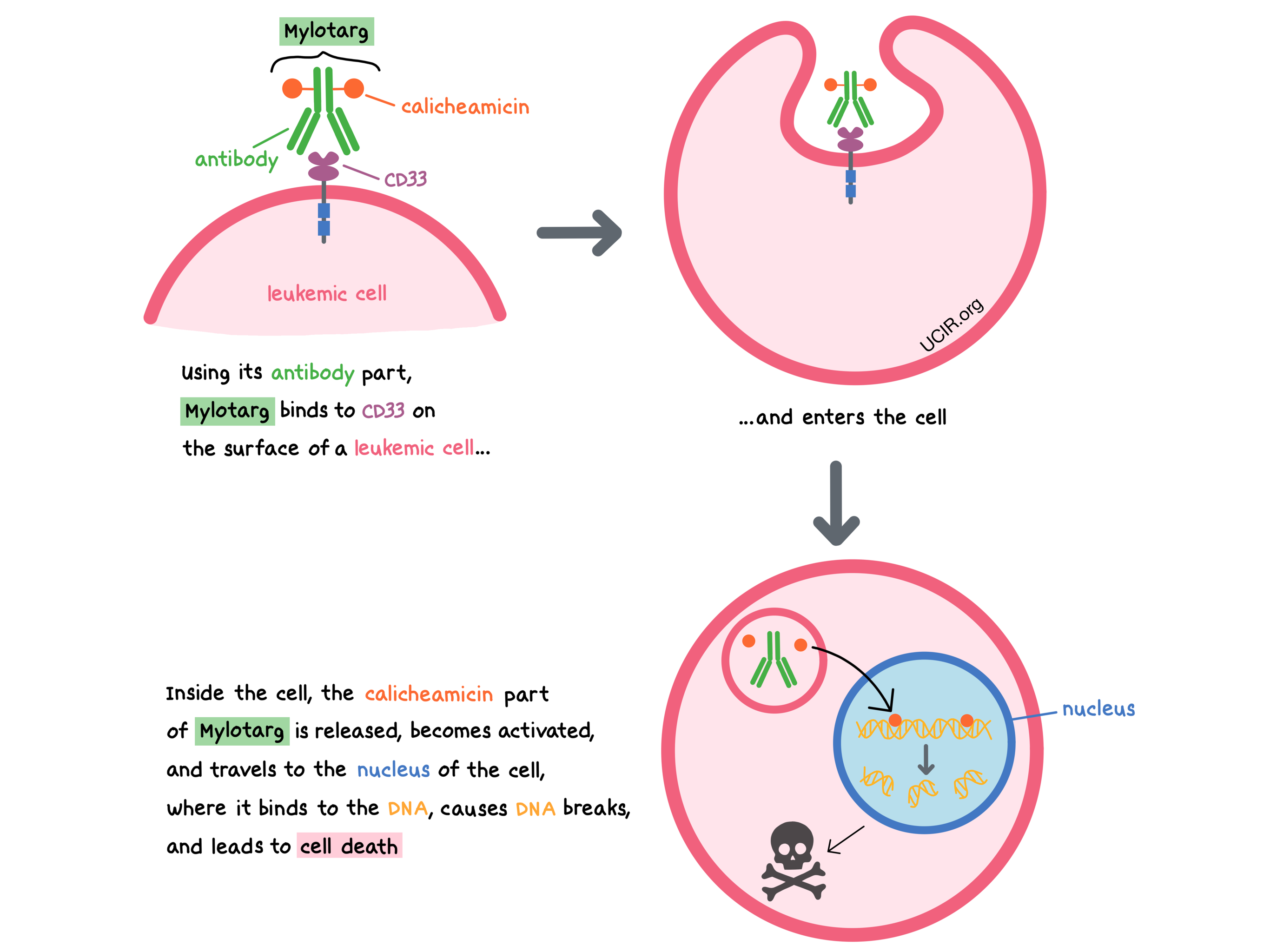
Mylotarg is a medicine that consists of two parts that are connected: an antibody that attaches to a molecule called CD33 on the surface of acute myeloid leukemia (AML) cells, and a drug called calicheamicin that can kill the cancer cells.
CD33 is present on the surface of certain blood-forming cells in the bone marrow and some mature cells present in the blood (myeloid cells, such as monocytes and mast cells). In a healthy person, it plays a role in interactions between cells. CD33 is also present on the surface of most AML cells. By targeting CD33, Mylotarg is designed to minimize harm to normal, healthy cells and to bring the cell-killing drug directly to the leukemic cells. When Mylotarg attaches to CD33 and enters the leukemic cell, the cell-killing drug part of Mylotarg, calicheamicin, is released, becomes activated, and travels to the nucleus of the cell, where it binds to the leukemic cell’s DNA, causes DNA breaks, and leads to cell death.
How is this drug given to the patient?
Prior to each administration of Mylotarg, patients receive several medications to help reduce the chance of a reaction to the infusion:
- Diphenhydramine (an antihistamine) one hour prior to receiving Mylotarg
- Acetaminophen one hour prior to receiving Mylotarg
- A corticosteroid 30 minutes prior to receiving Mylotarg
Mylotarg is administered via a tube into a vein (intravenous infusion, or I.V.) over two hours.
Patients with a high amount of cancer in their body may receive additional treatment (“prophylaxis”) prior to receiving Mylotarg to prevent tumor lysis syndrome, a serious side effect caused by the rapid breakdown of cancerous cells.
For newly diagnosed acute myeloid leukemia, Mylotarg may be administered with or without chemotherapy. For the combination treatment, adult patients receive the chemotherapies daunorubicin and cytarabine over a 1-week period, and Mylotarg is administered on days 1, 4, and 7 of the same week. This 7-day period is one cycle, and this first cycle is called an induction cycle. If another induction cycle is required, chemotherapy is administered without Mylotarg. If the patient responds to induction, the patient may receive consolidation cycles. During a consolidation cycle, Mylotarg is administered with daunorubicin and cytarabine on day 1.
When Mylotarg is given without chemotherapy for newly diagnosed acute myeloid leukemia, it is administered on days 1 and 8 of the induction cycle, and then once every 4 weeks on day 1 for up to 8 cycles.
For previously treated acute myeloid leukemia, Mylotarg is administered by itself over a single treatment course, on days 1, 4, and 7.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies.
Newly diagnosed CD33-positive acute myeloid leukemia
In a clinical trial, 271 adult patients (aged 50-70 years) with newly diagnosed, previously untreated acute myeloid leukemia that tested positive for the CD33 molecule were treated with either:
- Mylotarg, daunorubicin, and cytarabine, OR
- daunorubicin and cytarabine
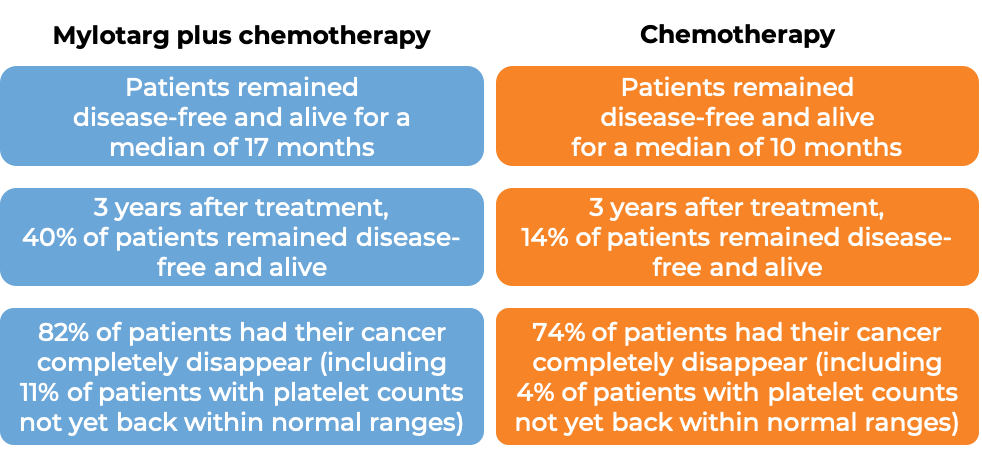
Patients treated with Mylotarg, daunorubicin, and cytarabine remained disease-free and alive for a median of 17 months, compared to 10 months for patients treated with daunorubicin and cytarabine alone. Patients with a response to treatment were allowed to receive a stem cell transplant, but it was recommended to delay stem cell transplantation by at least 2 months following Mylotarg treatment.
In another clinical trial, 1063 patients (aged 0-29 years) with newly diagnosed acute myeloid leukemia were treated with either Mylotarg plus chemotherapy or chemotherapy alone. Five years after treatment, 48% of patients treated with Mylotarg plus chemotherapy remained disease-free and alive, compared to 40% of patients treated with chemotherapy alone. Patients with a response to treatment were allowed to receive a stem cell transplant.
In another clinical trial, 237 patients (aged 62-88 years) with newly diagnosed acute myeloid leukemia that tested positive for the CD33 molecule, and who were not receiving intensive chemotherapy, were treated with either Mylotarg or best supportive care. Patients treated with Mylotarg lived for a median of 5 months, compared to 4 months for patients receiving best supportive care.
Previously treated CD33-positive acute myeloid leukemia
In a clinical trial, 57 adult patients with acute myeloid leukemia that tested positive for the CD33 molecule, and who had received prior treatment for their disease, and the disease came back (relapsed disease), or the treatment either did not work or stopped working (refractory disease), were treated with Mylotarg. Patients were allowed to receive a stem cell transplant after treatment with Mylotarg, but it was recommended to delay stem cell transplantation by at least 90 days following Mylotarg. 26% of patients treated with Mylotarg had their disease completely disappear. Patients remained disease-free for a median of 12 months.
What are the side effects?
The most common side effects of Mylotarg include fever, infection, bleeding, nausea, vomiting, constipation, rash, headache, liver problems, mouth sores, inflammation of the gut, and low white blood cell count (with or without fever).
Mylotarg can cause side effects that can become serious or life-threatening and may lead to death. Some of the serious side effects related to Mylotarg include problems with the liver or the heart, severe bleeding, and reactions related to the infusion (including difficulty breathing). Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
Severe liver problems
Mylotarg may cause severe, life-threatening, or fatal liver problems, including a condition called veno-occlusive disease (VOD). VOD occurs when small blood vessels that lead into the liver or are inside the liver become blocked. VOD can occur during treatment with Mylotarg or during a subsequent stem cell transplant. Symptoms of VOD may include rapid weight gain, abdominal swelling that may be painful, and yellowing of the whites of the eyes.
Patients may be at a higher risk of VOD if they:
- Have received higher doses of Mylotarg
- Have a history of moderate or severe liver problems
- Have previously received a stem cell transplant
- Receive a stem cell transplant after being treated with Mylotarg
Treatment with Mylotarg should be permanently discontinued if VOD occurs.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Pfizer
Approval
FDA and EMA
Links to drug websites
- US: https://www.pfizerpro.com/product/mylotarg
- Europe: https://www.ema.europa.eu/en/medicines/human/EPAR/mylotarg-0
Last updated March 11, 2022