How is the drug name pronounced?
Enfortumab Vedotin: En-fort-TU-mu-MAB ved-AUGHT-in
Padcev: PAD-sev
What cancer(s) does this drug treat?
Bladder and urinary tract cancer
Padcev is approved for:
- Patients with bladder or urinary tract cancer (urothelial cancer) that has grown or spread to other parts of the body, who have not yet received treatment for their advanced disease. In such cases, Padcev is used in combination with pembrolizumab (Keytruda).
-
Patients with bladder and urinary tract cancer (urothelial cancer) that has grown or spread to other parts of the body, and
- who have previously received a PD-1 or PD-L1 inhibitor, and a platinum-containing chemotherapy, OR
-
who have been previously treated and are not suited to receive cisplatin-containing chemotherapy.
In such cases, Padcev may be used by itself.
Limitations of Use
Age: The safety and efficacy of Padcev has not been established in patients under 18 years of age.
Fertility/Pregnancy/breastfeeding: Padcev may impair fertility permanently in men and temporarily in women. Padcev can cause harm to the fetus, and is not recommended for use during pregnancy. Women should not start Padcev treatment when pregnant. Women are advised to use contraception during treatment with Padcev and for at least 2 months after the last dose of Padcev. Men are advised to use contraception during treatment with Padcev and for at least 4 months after the last dose of Padcev. The risks associated with Padcev during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Padcev and for up to 3 weeks after the last dose of Padcev.
Exclusions: Padcev is not recommended for patients with moderate to severe liver disease.
Interactions with other drugs: Patients who are treated with medications called strong dual CYP3A4 and P-gp inhibitors during Padcev treatment should be closely monitored for signs of adverse reactions.
What type of immunotherapy is this?
- Antibody–drug conjugate
How does this drug work?
Target:
- Nectin-4
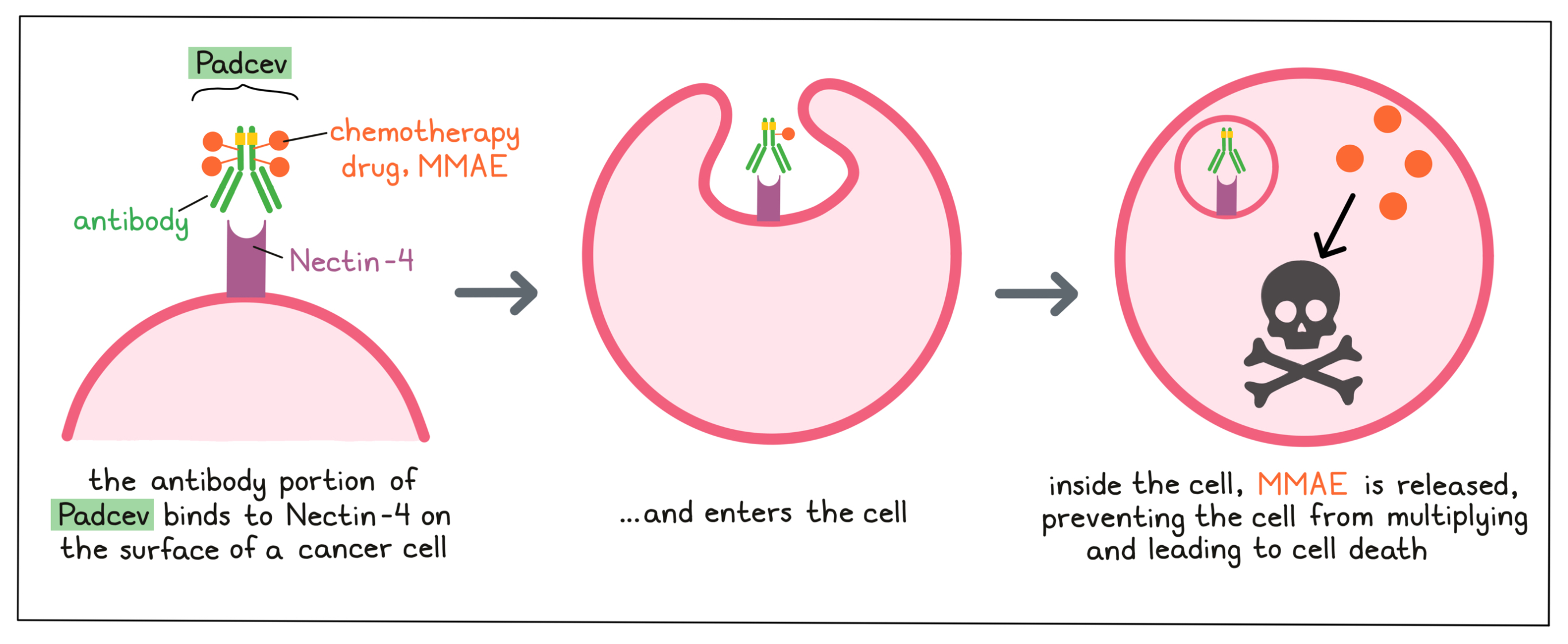
Padcev is an antibody–drug conjugate that was made in the laboratory by joining a small, highly potent chemotherapy to a large antibody molecule. The antibody delivers the chemotherapy drug to a cancer cell, reducing the effects of the chemotherapy drug on most other cells of the body.
Antibody molecules are proteins that can bind tightly to a particular target molecule. Antibodies have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. The target molecule that the antibody in Padcev binds to is Nectin-4, a molecule that is found in high amounts on the surface of urothelial cancer cells.
The chemotherapy drug Monomethyl auristatin E (MMAE) is joined to the stem of the antibody’s “Y” shape. There are about 4 MMAE molecules bound to every antibody molecule in Padcev. MMAE is not toxic when it is joined to the antibody, and needs to be released in order to become active.
When Padcev binds to Nectin-4 on the surface of cancer cells, it can enter the cell it is bound to. Inside the cell, the chemotherapy part of Padcev is released and becomes activated. MMAE prevents the cancer cells from multiplying and leads to their death.
Because Nectin-4 is also present on healthy cells of the urethra and bladder and on some other cell types, Padcev may attack these cells and cause unwanted effects, in addition to killing cancer cells.
How is this drug given to the patient?
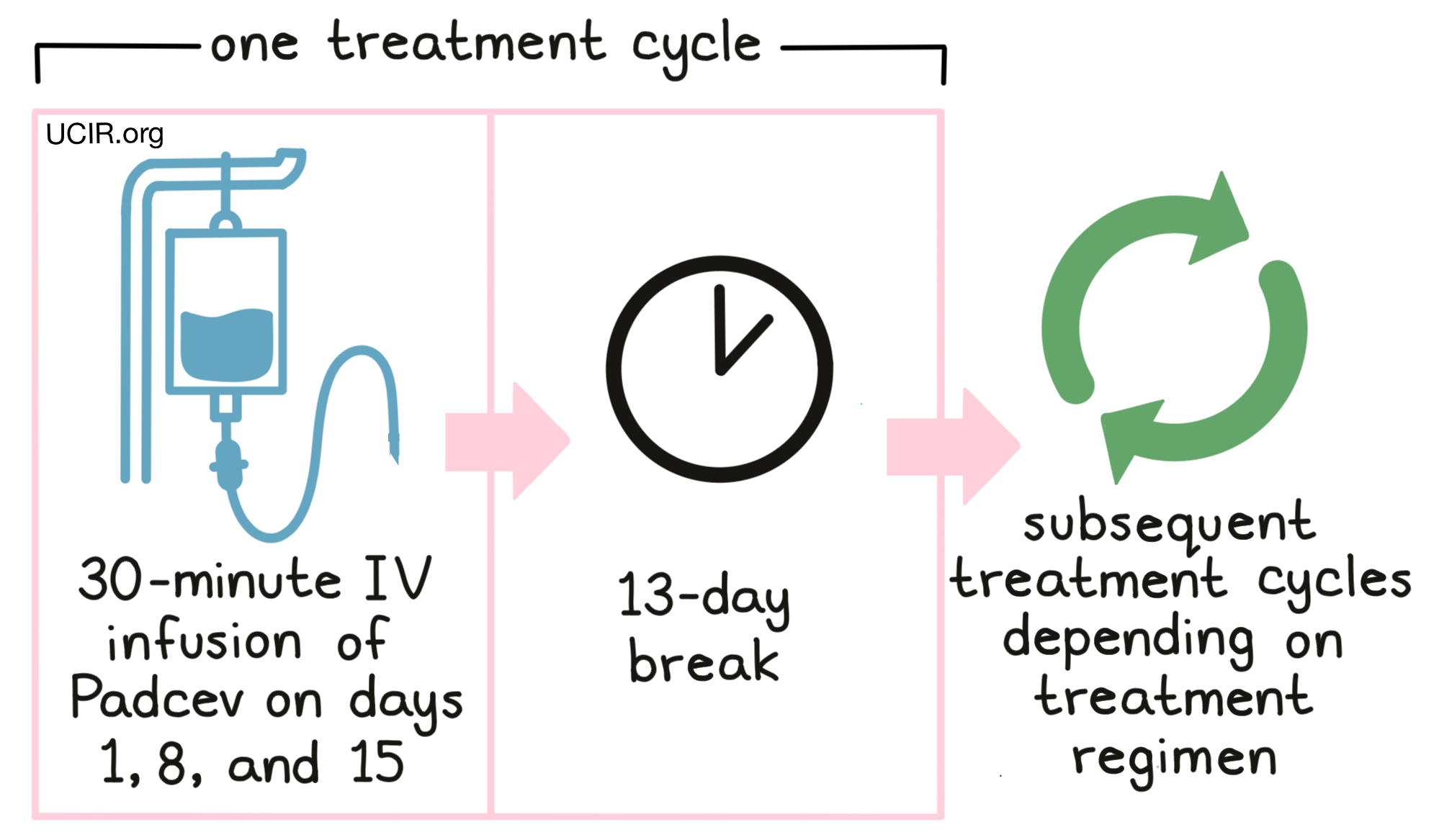
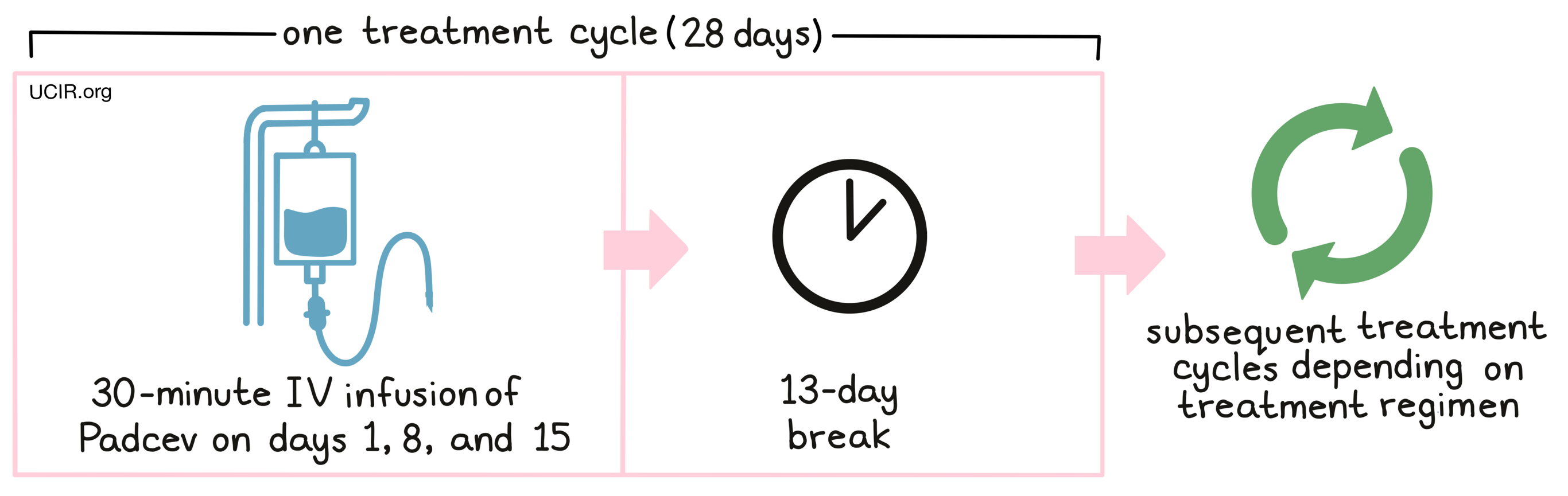
Padcev as a single agent is administered via a tube in the vein (intravenous infusion, or IV) over 30 minutes once a week for 3 weeks on days 1, 8 and 15, followed by 13 days with no treatment. Each 28-day (4-week) period is one “treatment cycle”.
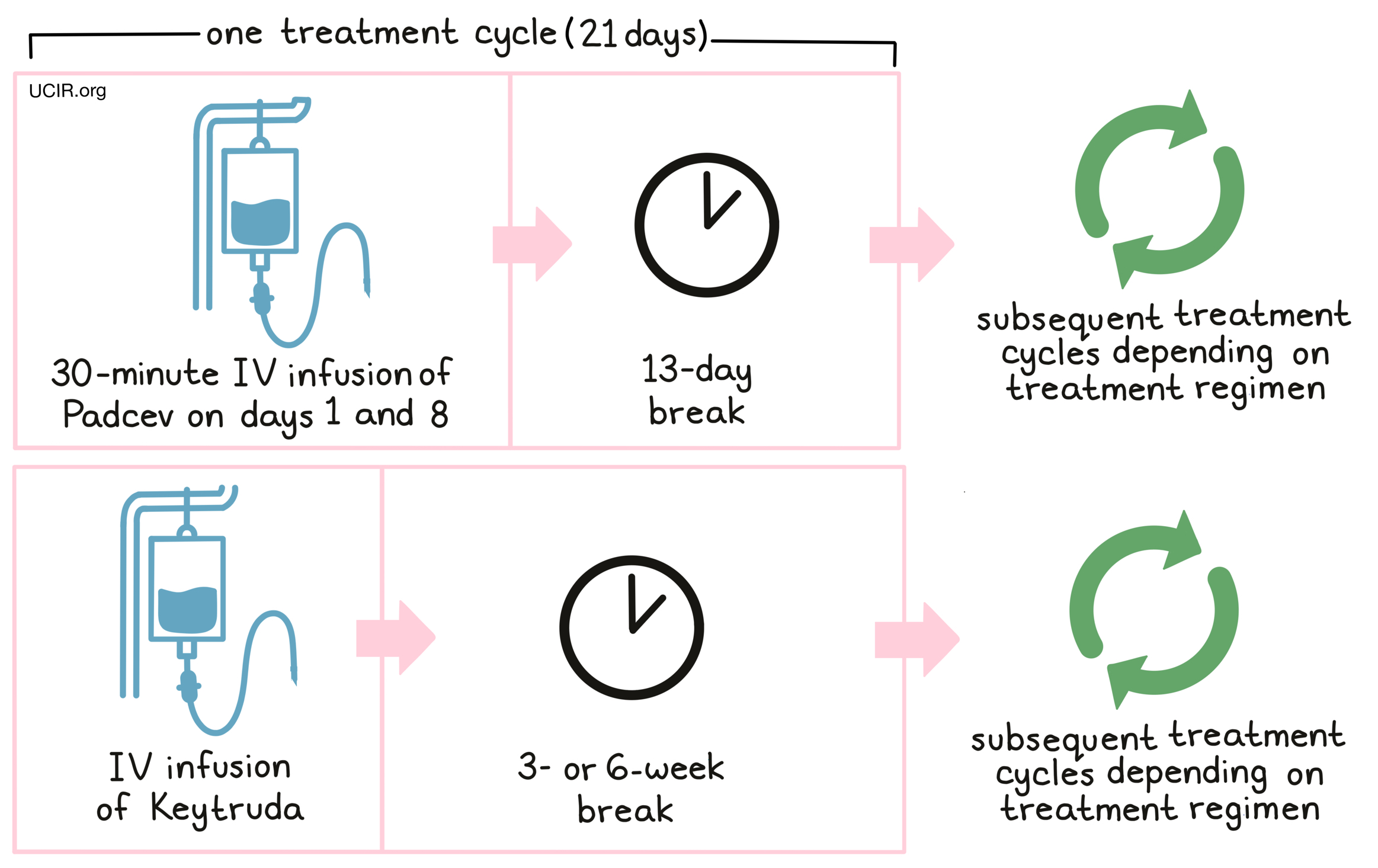
When given in combination with pembrolizumab (Keytruda), Padcev is administered via a tube in the vein (IV) over 30 minutes once a week for 2 weeks on days 1 and 8, followed by 13 days with no treatment. In this case, each treatment cycle is 21 days (3 weeks) long. Pembrolizumab (Keytruda) is given via IV every 3 or 6 weeks (depending on the dose) after the administration of Padcev when given on the same day.
The number of treatment cycles the patient receives is determined by their healthcare provider, and is based on the response to treatment.
What are the observed clinical results?
For:
Bladder and urinary tract cancer
previously untreated
previously treated with a PD-1 or PD-L1 inhibitor and platinum-containing chemotherapy
previously treated with a PD-1 or PD-L1 inhibitor and not suited for treatment with cisplatin-containing chemotherapy
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Bladder and urinary tract cancer (previously untreated)
In a clinical trial, 121 patients with bladder and urinary tract cancer (urothelial cancer) that had grown or spread to other parts of the body, who had not received treatment for their advanced disease, and who were not suited to receive cisplatin-containing chemotherapy, were treated with Padcev and pembrolizumab (Keytruda).
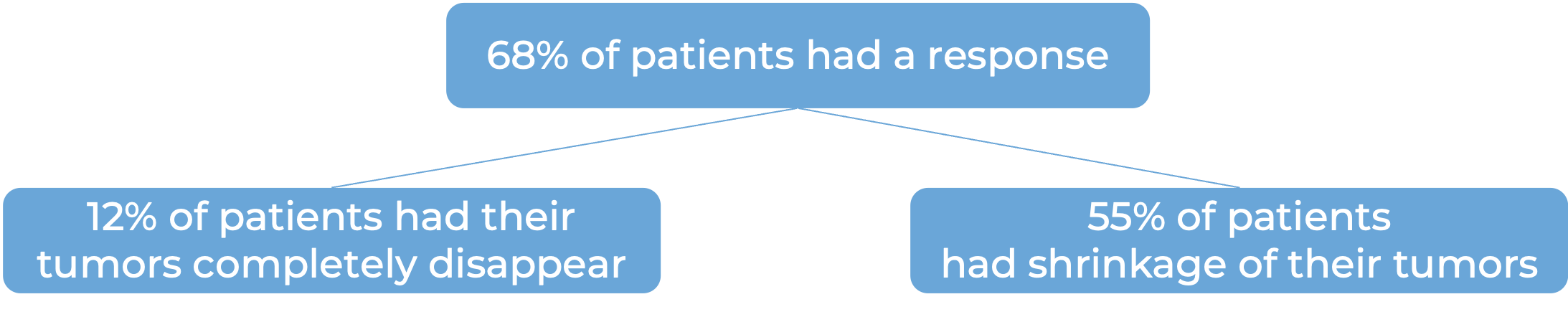
In another clinical trial, 886 patients with bladder and urinary tract cancer (urothelial cancer) that had grown or spread to other parts of the body, who had not received treatment for their advanced disease, were treated with Padcev plus pembrolizumab (Keytruda) or with standard chemotherapy (gemcitabine and cisplatin).
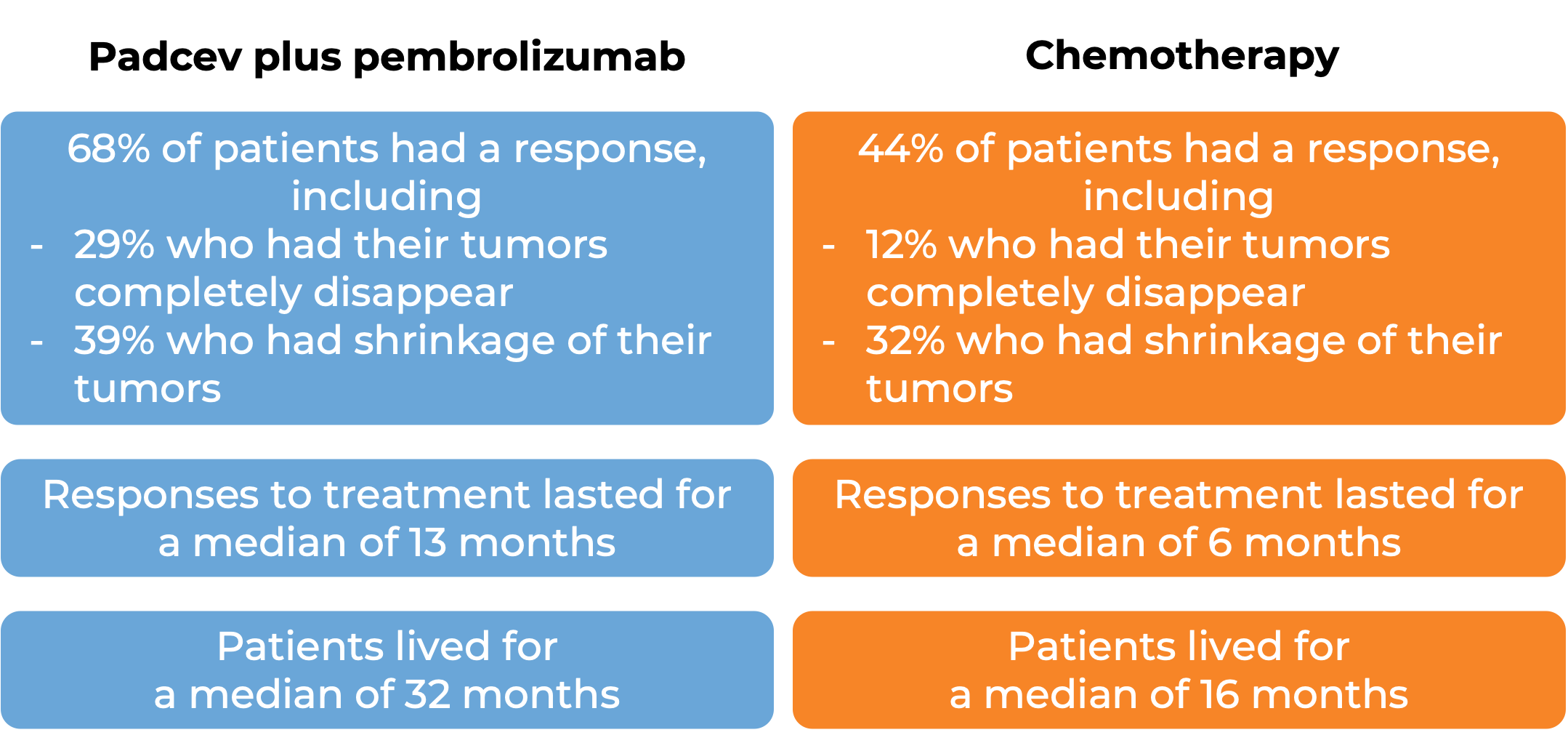
(For the definition of “median”, click HERE.)
Bladder and urinary tract cancer (previously treated with a PD-1 or PD-L1 inhibitor and platinum-containing chemotherapy)
In a clinical trial, 608 patients with bladder and urinary tract cancer (urothelial cancer) that had grown or spread to other parts of the body, and who had previously received a PD-1 or PD-L1 inhibitor, and a platinum-containing chemotherapy, were treated with either Padcev or the investigators choice of chemotherapy (docetaxel, paclitaxel, or vinflunine).
At a median follow-up of 11 months:
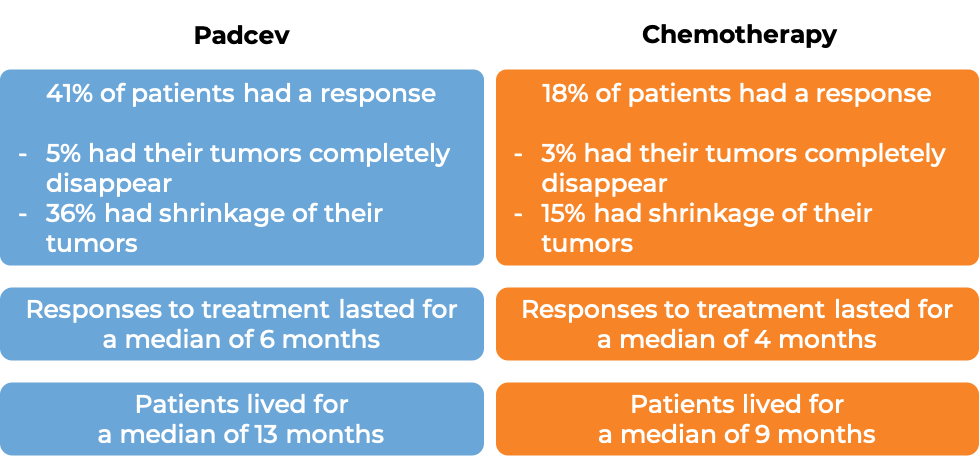
In another clinical trial, 125 patients with bladder and urinary tract cancer (urothelial cancer) that had grown or spread to other parts of the body, and who had previously received a PD-1 or PD-L1 inhibitor, and a platinum-containing chemotherapy, were treated with Padcev.
At a median follow-up of 10 months:
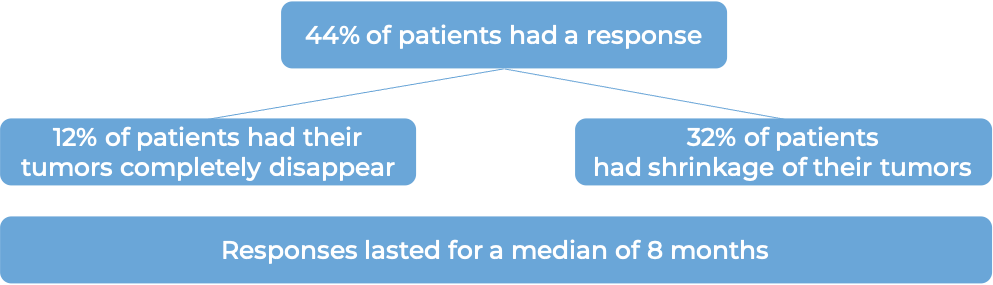
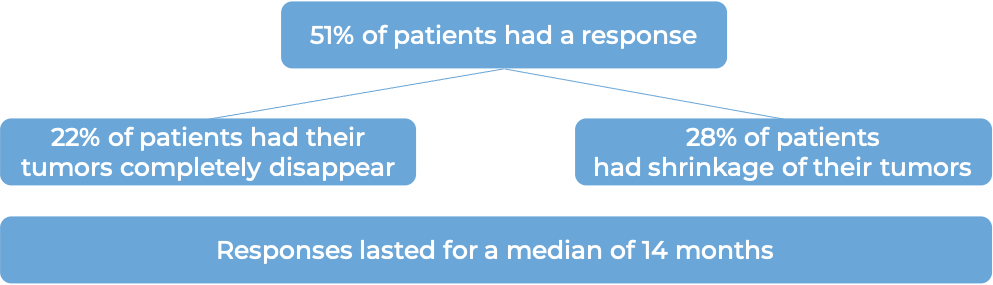
Bladder and urinary tract cancer (previously treated with a PD-1 or PD-L1 inhibitor and not suited for treatment with cisplatin-containing chemotherapy)
In a clinical trial, 89 patients with previously treated bladder and urinary tract cancer (urothelial cancer) that had grown or spread to other parts of the body, and who could not be treated with cisplatin-containing chemotherapy, were treated with Padcev.
At a median follow-up of 13 months:
What are the side effects?
The most common side effects of Padcev include nausea, diarrhea, changes in sense of taste, fatigue, decreased appetite, decreased weight, hair loss, dry eyes and skin, rash, numbness or tingling in the hands or feet, muscular weakness, changes in liver and kidney function test results, and abnormal blood test results.
Other potential side effects of Padcev treatment include high blood sugar, eye problems (e.g., blurred vision, pink eye, dry eye), and leakage of Padcev from the vein it was administered to into the surrounding tissue. Patients should immediately report burning, pain, stinging, redness, or swelling around the injection or infusion site to their healthcare provider.
Padcev can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Padcev are skin reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. These skin reactions typically develop during the first treatment cycle. Stevens-Johnson syndrome may initially present with flu-like symptoms, progressing into a widespread, blistering rash. As the condition progresses, the affected areas of the skin may begin to die and slough off. Toxic epidermal necrolysis is characterized by fever and reddening of the skin. The skin is often painful to the touch, and begins to blister and die, sloughing off. Padcev may also cause inflammation and scarring of the lungs (pneumonitis/interstitial lung disease).
Patients should immediately report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer
Astellas Pharma and Seattle Genetics
Approval
FDA and EMA
Links to drug websites
Other references:
- Enfortumab Vedotin in Previously treated Advanced Urothelial Carcinoma. Powles T, et al. New England Journal of Medicine (2021)
Last updated: March 22, 2024