How is this drug name pronounced?
Elotuzumab: EH-loh-TOO-zoo-mab
Empliciti: em-PLIH-sih-tee
What cancer(s) does this drug treat?
Multiple myeloma
Empliciti is approved for:
- Patients with multiple myeloma who have received one to three prior therapies for their disease. In such cases, Empliciti is used in combination with lenalidomide (Revlimid) and dexamethasone.
- Patients with multiple myeloma who have received at least two prior therapies for their disease, including lenalidomide (Revlimid) and a proteasome inhibitor. In such cases, Empliciti is used in combination with pomalidomide (Pomalyst) and dexamethasone.
Limitations of use:
Age: The safety and efficacy of Empliciti in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: When Empliciti is used in combination with lenalidomide (Revlimid) or pomalidomide (Pomalyst), the treatment can cause harm to a fetus, including severe, life-threatening birth defects, and is not recommended for use during pregnancy. Women and men are advised to refer to the lenalidomide and pomalidomide prescribing information for contraception requirements. The risks associated with Empliciti during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Empliciti.
What type of immunotherapy is this?
How does this drug work?
- Target: SLAMF7 (Signaling Lymphocytic Activation Molecule Family Member 7)
Empliciti is an antibody that was made in the laboratory. Empliciti and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. These two upper arms of Empliciti attach to a molecule called SLAMF7 (Signaling Lymphocytic Activation Molecule Family Member 7). SLAMF7 is present on the surface of many types of immune cells, including natural killer (NK) cells, and it plays a role in regulating a normal immune response. SLAMF7 is also found in high amounts on the surface of myeloma cells. Further, the stem of Empliciti’s “Y” shape has binding sites for immune cells or other parts of the immune system.
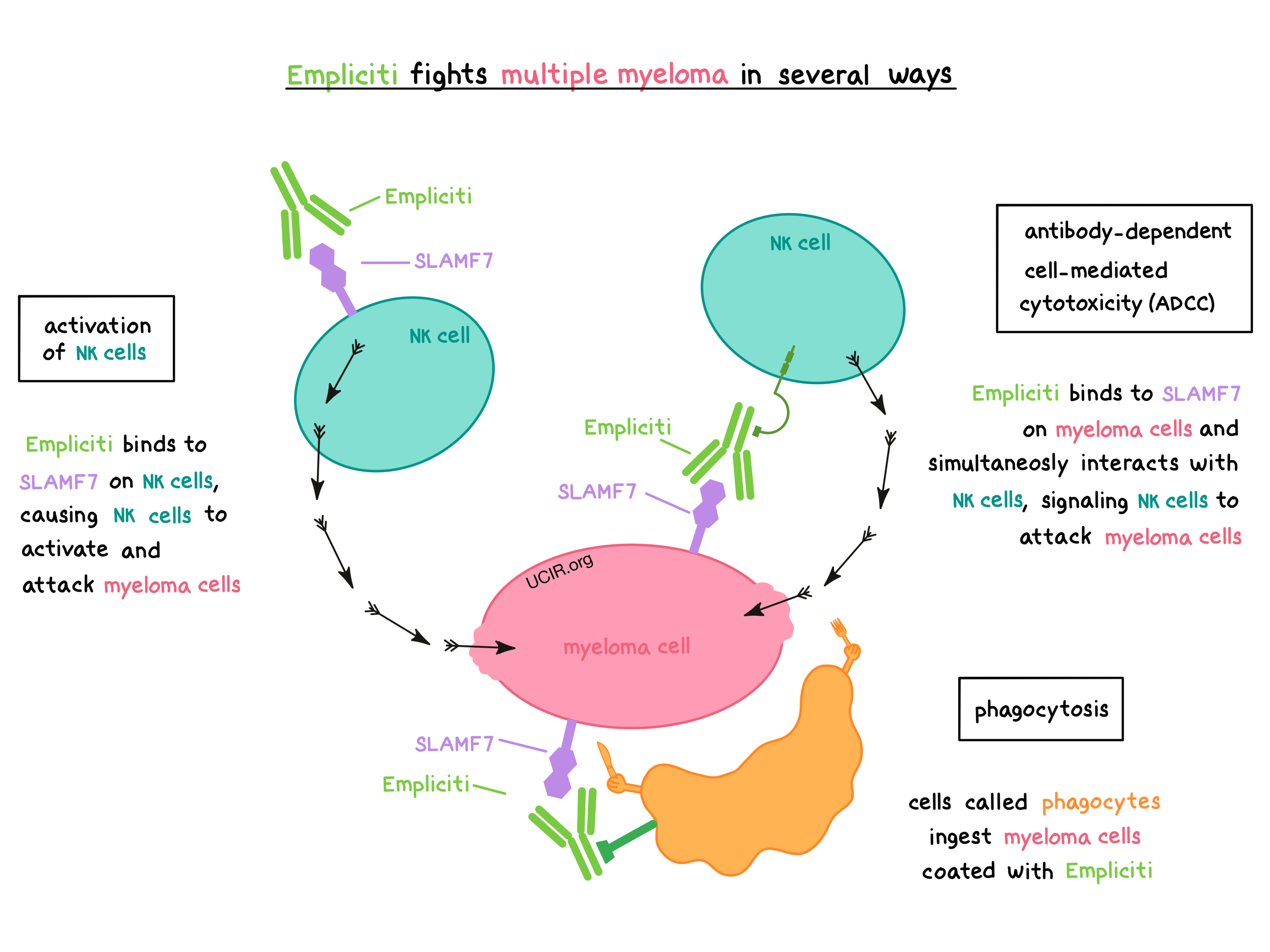
Empliciti works in at least three ways to help fight multiple myeloma.
Activation of NK cells
Empliciti binds to SLAMF7 on the surface of NK cells, causing the NK cells to become activated and attack myeloma cells.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to SLAMF7 on the surface of myeloma cells, the “stem” of Empliciti can also attract and bind immune cells such as NK cells. This allows Empliciti to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell that Empliciti is bound to.
Phagocytosis
When Empliciti is bound to myeloma cells, it can also attract immune cells called phagocytes, which have the ability to ingest (“eat”) cells that have been coated with Empliciti.
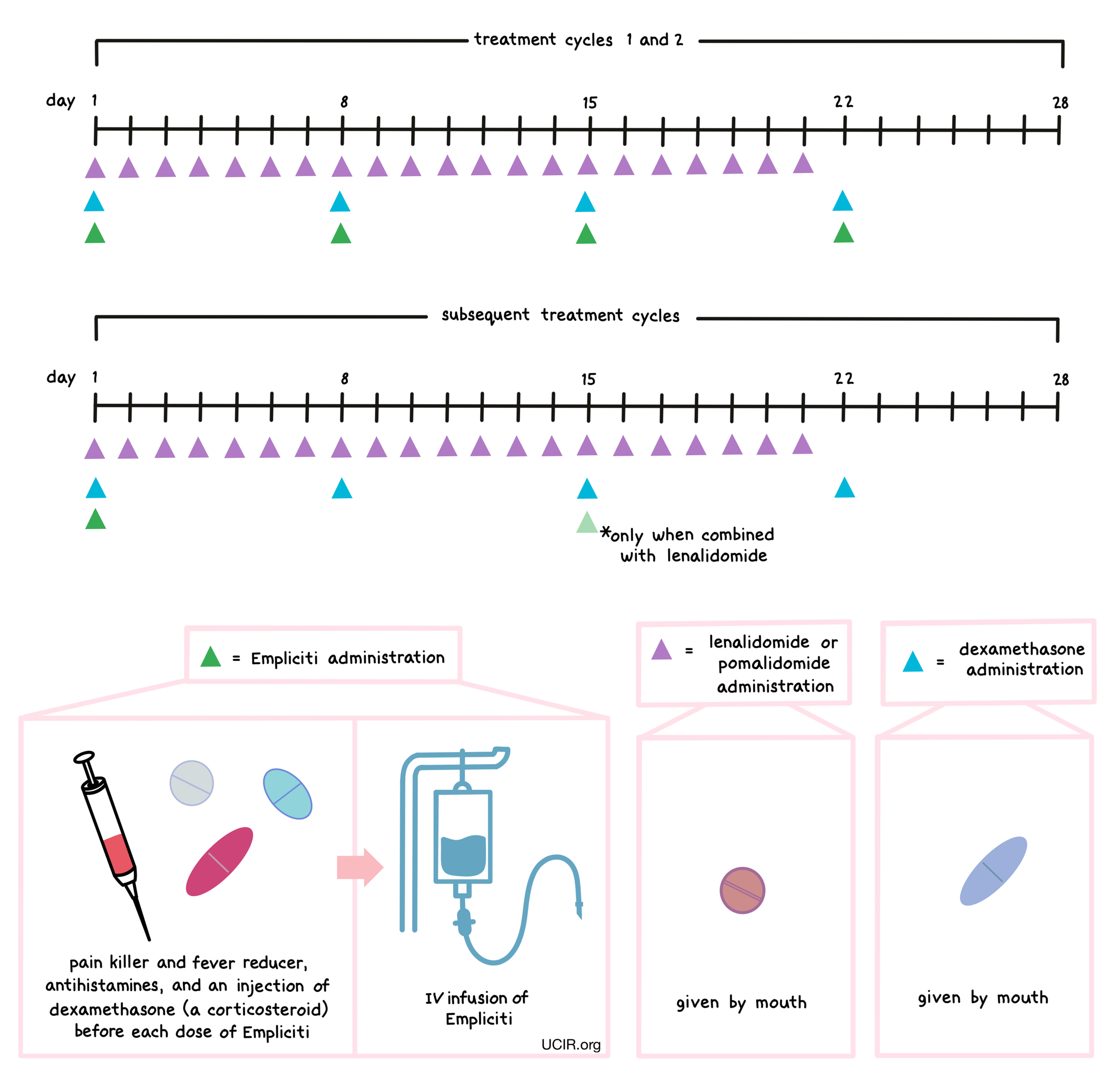
How is this drug given to the patient
About 45 to 90 minutes prior to receiving each dose of Empliciti, patients receive several medications to help reduce the chance of a reaction to the infusion:
- An H1 blocker antihistamine (such as diphenhydramine; delivered orally or into a vein)
- An H2 blocker antihistamine (such as ranitidine; delivered orally or into a vein)
- A painkiller and fever reducer (acetaminophen; delivered orally)
- A corticosteroid (dexamethasone; delivered into a vein)
Empliciti is administered via a tube into a vein (intravenous infusion, or I.V.) several times during a 28-day “treatment cycle”. During the first two treatment cycles, Empliciti is administered once a week. During all subsequent treatment cycles, Empliciti is administered every two or four weeks, depending on the treatment combination. The duration of the infusion depends on the patient’s body weight and how many previous doses of Empliciti have been administered. Infusion of Empliciti does not require a hospital stay.
Empliciti is administered in combination with lenalidomide (Revlimid) or pomalidomide (Pomalyst) and dexamethasone. Lenalidomide or pomalidomide is given by mouth once daily from day 1 to day 21 of each 28-day treatment cycle. Dexamethasone is also given by mouth (in addition to the dose of dexamethasone that is injected into a vein before Empliciti infusion)on days 1, 8, 15, and 22 for each 28-day cycle.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Multiple myeloma
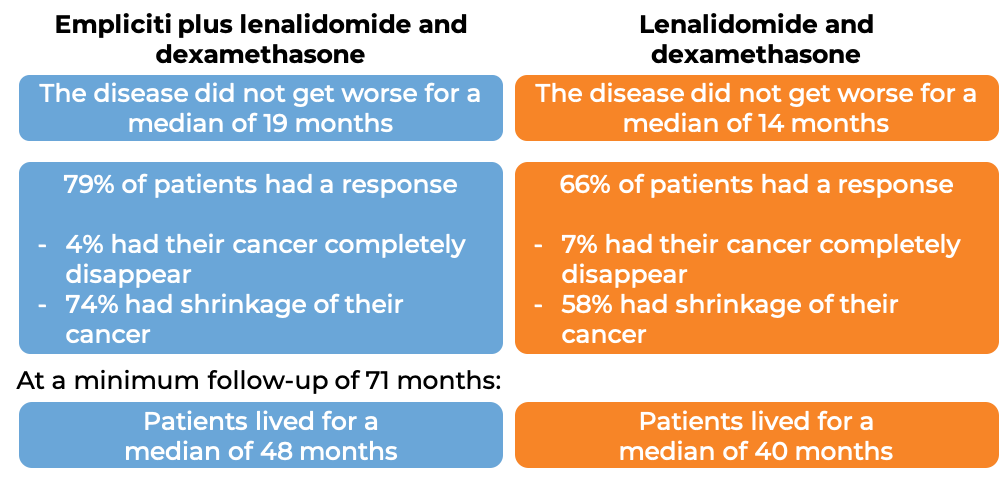
In a clinical trial, 646 patients with multiple myeloma who had received one to three prior therapies for their disease, and the most recent therapy either did not work or stopped working, were treated with either:
- Empliciti plus lenalidomide (Revlimid) and dexamethasone, OR
- lenalidomide (Revlimid) and dexamethasone.
At a median follow-up of 25 months:
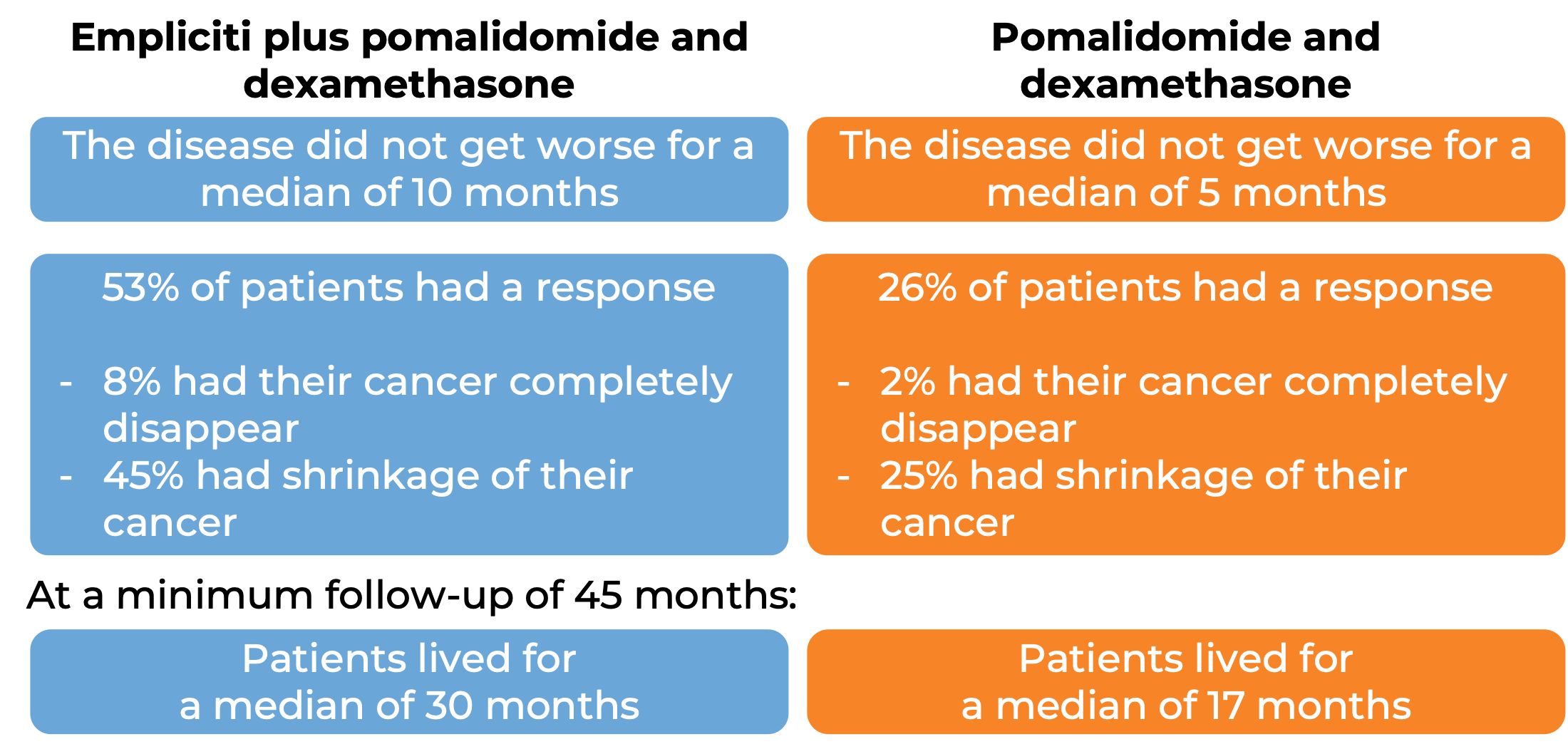
In another clinical trial, 117 patients with multiple myeloma who had received at least two prior treatments (including lenalidomide [Revlimid] and a proteasome inhibitor), and whose disease had either come back after or had not responded to the last treatment, were treated with either:
- Empliciti plus pomalidomide (Pomalyst) and dexamethasone, OR
- pomalidomide (Pomalyst) and dexamethasone.
At a minimum follow-up of 9 months:
What are the side effects?
The most common side effects of Empliciti include fatigue, fever, cough, sore throat, runny nose, upper respiratory tract infection, pneumonia, diarrhea, constipation, decreased appetite, weight loss, high blood sugar, low white blood cell count, and numbness, weakness, tingling, or burning pain in the arms or legs.
Empliciti can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Empliciti include serious infections, blood clots, risk of developing new cancers, and problems with the liver. Reactions related to the infusion may also occur.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Bristol-Myers Squibb
Approval
FDA and EMA
Links to drug websites
Other references:
Last updated January 27, 2023
How is this drug name pronounced?
Elotuzumab: EH-loh-TOO-zoo-mab
Empliciti: em-PLIH-sih-tee
What cancer(s) does this drug treat?
Multiple myeloma
Empliciti is approved for:
- Patients with multiple myeloma who have received at least one prior therapy for their disease. In such cases, Empliciti is used in combination with lenalidomide (Revlimid) and dexamethasone.
- Patients with multiple myeloma who have received at least two prior therapies for their disease, including lenalidomide (Revlimid) and a proteasome inhibitor, and the treatment either did not work or stopped working. In such cases, Empliciti is used in combination with pomalidomide (Pomalyst) and dexamethasone.
Limitations of use:
Age: The safety and efficacy of Empliciti in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: When Empliciti is used in combination with lenalidomide (Revlimid) or pomalidomide (Pomalyst), the treatment can cause harm to a fetus, including severe, life-threatening birth defects, and is not recommended for use during pregnancy. Women are advised to use contraception during treatment with Empliciti. Men should use contraceptive methods during treatment with Empliciti and for 180 days after the end of treatment. Women and men are advised to also refer to the lenalidomide and pomalidomide prescribing information for contraception requirements. The risks associated with Empliciti during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Empliciti, particularly because of its use in combination with lenalidomide (Revlimid) or pomalidomide (Pomalyst).
What type of immunotherapy is this?
How does this drug work?
- Target: SLAMF7 (Signaling Lymphocytic Activation Molecule Family Member 7)
Empliciti is an antibody that was made in the laboratory. Empliciti and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. These two upper arms of Empliciti attach to a molecule called SLAMF7 (Signaling Lymphocytic Activation Molecule Family Member 7). SLAMF7 is present on the surface of many types of immune cells, including natural killer (NK) cells, and it plays a role in regulating a normal immune response. SLAMF7 is also found in high amounts on the surface of myeloma cells.
The stem of Empliciti’s “Y” shape has binding sites for immune cells or other parts of the immune system.
Empliciti works in at least three ways to help fight multiple myeloma.
Activation of NK cells
Empliciti binds to SLAMF7 on the surface of NK cells, causing the NK cells to become activated and attack the myeloma cells.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to SLAMF7 on the surface of myeloma cells, the “stem” of Empliciti can also attract and bind immune cells such as NK cells. This allows Empliciti to act as a bridge between the target cell and the immune cell. The immune cell then releases molecules that can kill the cell that Empliciti is bound to.
Phagocytosis
When Empliciti is bound to myeloma cells, it can also attract immune cells called phagocytes, which have the ability to ingest (“eat”) cells that have been coated with Empliciti

How is this drug given to the patient?
About 45 to 90 minutes prior to receiving each dose of Empliciti, patients receive several medications to help reduce the chance of a reaction to the infusion:
- An H1 blocker antihistamine (such as diphenhydramine; delivered orally or into a vein)
- An H2 blocker antihistamine (such as ranitidine; delivered orally or into a vein)
- A painkiller and fever reducer (acetaminophen; delivered orally)
- A corticosteroid (dexamethasone; delivered into a vein)
Empliciti is administered via a tube into a vein (intravenous infusion, or i.v.) several times during a 28-day “treatment cycle”. During the first two treatment cycles, Empliciti is administered every week, and every two or four weeks for all subsequent cycles, depending on the treatment combination. The duration of the infusion depends on the patient’s body weight and how many previous doses of Empliciti have been administered. Infusion of Empliciti does not require a hospital stay.
Empliciti is administered in combination with lenalidomide (Revlimid) or pomalidomide (Pomalyst) and dexamethasone. Lenalidomide or pomalidomide is given by mouth once daily from day 1 to day 21 of each 28-day treatment cycle. Low-dose dexamethasone (in addition to dexamethasone which is injected into a vein before Empliciti infusion) is given by mouth on days 1, 8, 15, and 22 of each 28-day cycle.

What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Multiple myeloma
In a clinical trial, 646 patients with multiple myeloma who had received one to three prior therapies for their disease, and the most recent therapy either did not work or stopped working, were treated with either:
- Empliciti plus lenalidomide (Revlimid) and dexamethasone, OR
- lenalidomide (Revlimid) and dexamethasone.
At a median follow-up of 25 months,

In another clinical trial, 117 patients with multiple myeloma who had received at least two prior treatments (including lenalidomide [Revlimid] and a proteasome inhibitor), and whose disease had either come back after or had not responded to the last treatment, were treated with:
- Empliciti plus pomalidomide (Pomalyst) and dexamethasone, OR
- Pomalidomide (Pomalyst) and dexamethasone.
At a minimum follow-up of 9 months:

(For the definition of “median”, click HERE.)
What are the side effects?
The most common side effects of Empliciti include fatigue, fever, cough, sore throat, runny nose, upper respiratory tract infection, pneumonia, diarrhea, constipation, decreased appetite, weight loss, high blood sugar, low white blood cell count, and numbness, weakness, tingling, or burning pain in the arms or legs.
Empliciti can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Empliciti include serious infections, blood clots, risk of developing new cancers, and problems with the liver. Reactions related to the infusion may also occur.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Bristol-Myers Squibb
Approval
FDA and EMA
Links to drug websites
Last update on January 25, 2023






