How is this drug name pronounced?
Daratumumab: DAYR-uh-TOOM-yoo-mab
Darzalex: DAR-zah-lex
What cancer(s) does this drug treat?
Multiple myeloma (newly diagnosed)
Darzalex is approved for:
- Patients with newly diagnosed multiple myeloma who cannot receive an autologous stem cell transplant (stem cell transplant that uses their own stem cells). In such cases, Darzalex is used in combination with lenalidomide (Revlimid) and dexamethasone, OR in combination with bortezomib (Velcade), melphalan, and prednisone.
- Patients with newly diagnosed multiple myeloma who can receive an autologous stem cell transplant. In such cases, Darzalex is used in combination with bortezomib (Velcade), thalidomide, and dexamethasone.
Multiple myeloma (previously treated)
Darzalex is approved for:
- Patients with multiple myeloma who have received at least one prior treatment for their disease, and it either did not work or stopped working (refractory disease) and the cancer has come back (relapsed disease). In such cases, Darzalex is used in combination with lenalidomide (Revlimid) and dexamethasone, OR in combination with bortezomib (Velcade) and dexamethasone.
- Patients with multiple myeloma who have received at least two prior treatments for their disease, including lenalidomide (Revlimid) and a proteasome inhibitor, and the treatment either did not work or stopped working and the cancer has come back. In such cases, Darzalex is used in combination with pomalidomide (Pomalyst) and dexamethasone.
- Patients with multiple myeloma who have received one to three prior treatments for their disease, and the treatment either did not work or stopped working and the cancer has come back. In such cases, Darzalex is used in combination with carfilzomib (Kyprolis) and dexamethasone.
- Patients with multiple myeloma who have received at least three prior treatments for their disease, including a proteasome inhibitor and an immunomodulatory agent, or for whom both a proteasome inhibitor and an immunomodulatory agent did not work. In such cases, Darzalex is used by itself.
Limitations of use:
Age: The safety and efficacy of Darzalex in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: The risks associated with Darzalex during pregnancy are not known and cannot be ruled out. Due to the potential for harm to the fetus, Darzalex is not recommended for use during pregnancy. Women are advised to use contraception during treatment with Darzalex and for at least 3 months after the last dose of Darzalex. The risks associated with Darzalex during breastfeeding are not known and cannot be ruled out. Due to the potential for adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Darzalex.
What type of immunotherapy is this?
How does this drug work?
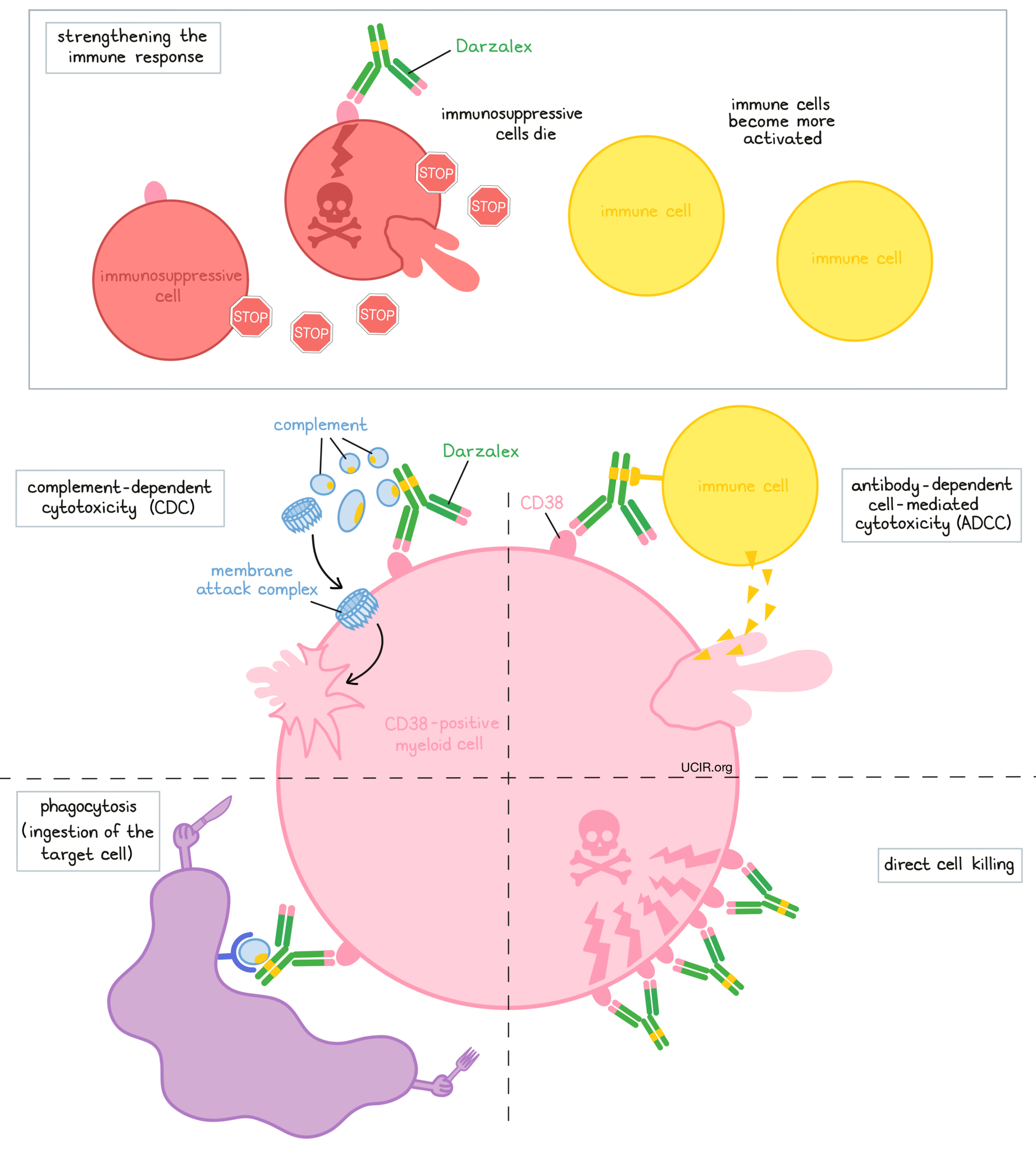
Darzalex is an antibody that was made in the laboratory. Darzalex and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. Darzalex attaches to a molecule called CD38. CD38 plays a role in the attachment of neighboring cells to each other, and in transmitting messages from outside of the cell to the inside of the cell. CD38 is present on the surface of many types of immune cells (white blood cells) and red blood cells, and is also found in high amounts on the surface of myeloma cells.
The stem of Darzalex’s “Y” shape has binding sites for immune cells or other parts of the immune system.
At least five different mechanisms are thought to be responsible for the elimination of myeloma cells by Darzalex. Darzalex works alone and/or is helped by the immune system to kill myeloma cells.
Direct cell killing
By binding to CD38 molecules on the surface of myeloma cells and bringing the CD38 molecules close together in “clusters”, Darzalex can directly cause the cells to die.
Complement-dependent cytotoxicity (CDC)
When bound to CD38 on the surface of myeloma cells, the “stem” of Darzalex can attract and bind molecules of the immune system called “complement” that freely flow in the blood or tissues. Activation of the complement system causes the formation of the “membrane attack complex”, which can puncture and destroy the cell that Darzalex is bound to.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to CD38 on the surface of myeloma cells, the “stem” of Darzalex can also attract and bind immune cells. This allows Darzalex to act as a bridge between the target cell and the immune cell (such as the natural killer (NK) cell). The immune cell then releases molecules that can kill the cell that Darzalex is bound to.
Phagocytosis
When Darzalex is bound to myeloma cells, it can also attract immune cells called phagocytes, which have the ability to ingest (“eat”) cells that have been coated with Darzalex.
Strengthening the immune response
Darzalex can bind to CD38 on the surface of certain types of immunosuppressive immune cells that reduce the strength of an immune response, causing such cells to die. At the same time Darzalex can activate other immune cells that are required for the elimination of cancer cells, thus allowing for a stronger immune attack on myeloma cells.
The combined effect of these mechanisms results in the elimination of melanoma from the body. Because CD38 is also found on certain normal cells, Darzalex can attack and reduce the numbers of some types of immune cells, including healthy B cells and natural killer (NK) cells.
How is this drug given to the patient?
About 1 to 3 hours prior to receiving each dose of Darzalex, patients receive several medications to help reduce the chance of a reaction to the infusion:
- A corticosteroid (dexamethasone or methylprednisolone)
- An antihistamine (diphenhydramine)
- A painkiller and fever reducer (acetaminophen)
Darzalex is administered via a tube into a vein (intravenous infusion, or i.v.) every one, two, three, or four weeks, depending on the treatment regimen and how long the patient has been receiving Darzalex. The first infusion of Darzalex may take on average about 7 hours, although the first dose may be split between two shorter infusions over two consecutive days. Subsequent infusions typically take 3 to 5 hours to administer. In most cases Darzalex is administered in combination with other medications. In such cases, a corticosteroid may be part of the treatment regimen in addition to the corticosteroid that is given before Darzalex infusion.
Patients may receive corticosteroids after each infusion of Darzalex to reduce the risk of a delayed reaction to the infusion.
Patients will receive antiviral medication to prevent herpes zoster (shingles) reactivation, beginning within 1 week of starting Darzalex and continuing for 3 months after treatment.
What are the observed clinical results?
For:
Multiple myeloma (newly diagnosed)
Multiple myeloma (previously treated)
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Multiple myeloma (newly diagnosed)
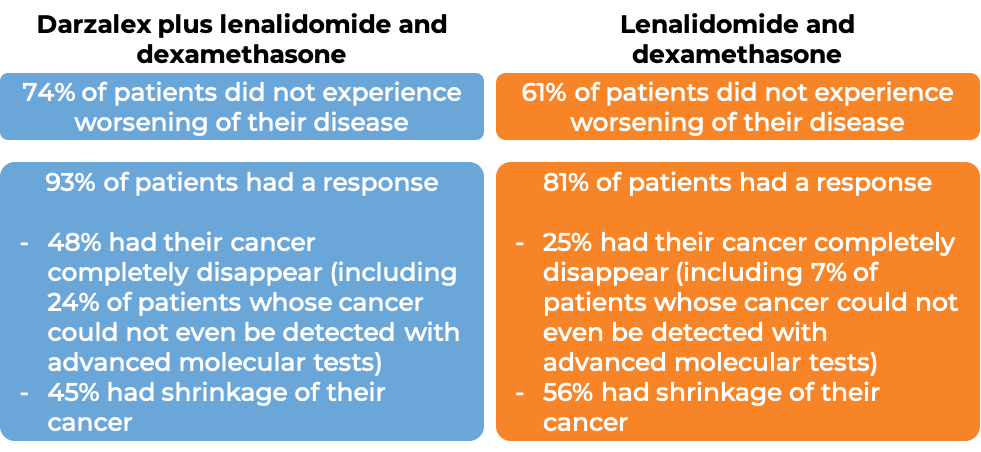
In a clinical trial, 737 patients with newly diagnosed multiple myeloma, who could not receive an autologous stem cell transplant (stem cell transplant that uses their own stem cells), were treated with either:
- Darzalex, lenalidomide (Revlimid), and low-dose dexamethasone, OR
- lenalidomide and low-dose dexamethasone
At a median follow-up of 28 months:
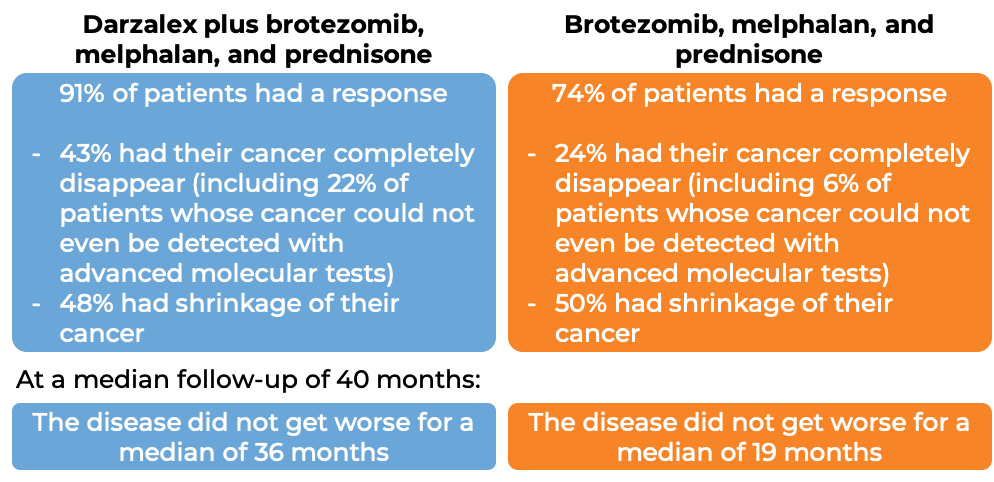
In another clinical trial, 706 patients with newly diagnosed multiple myeloma, who could not receive an autologous stem cell transplant, were treated with either:
- Darzalex, bortezomib (Velcade), melphalan, and prednisone, OR
- bortezomib (Velcade), melphalan, and prednisone
At a median follow-up of 17 months:
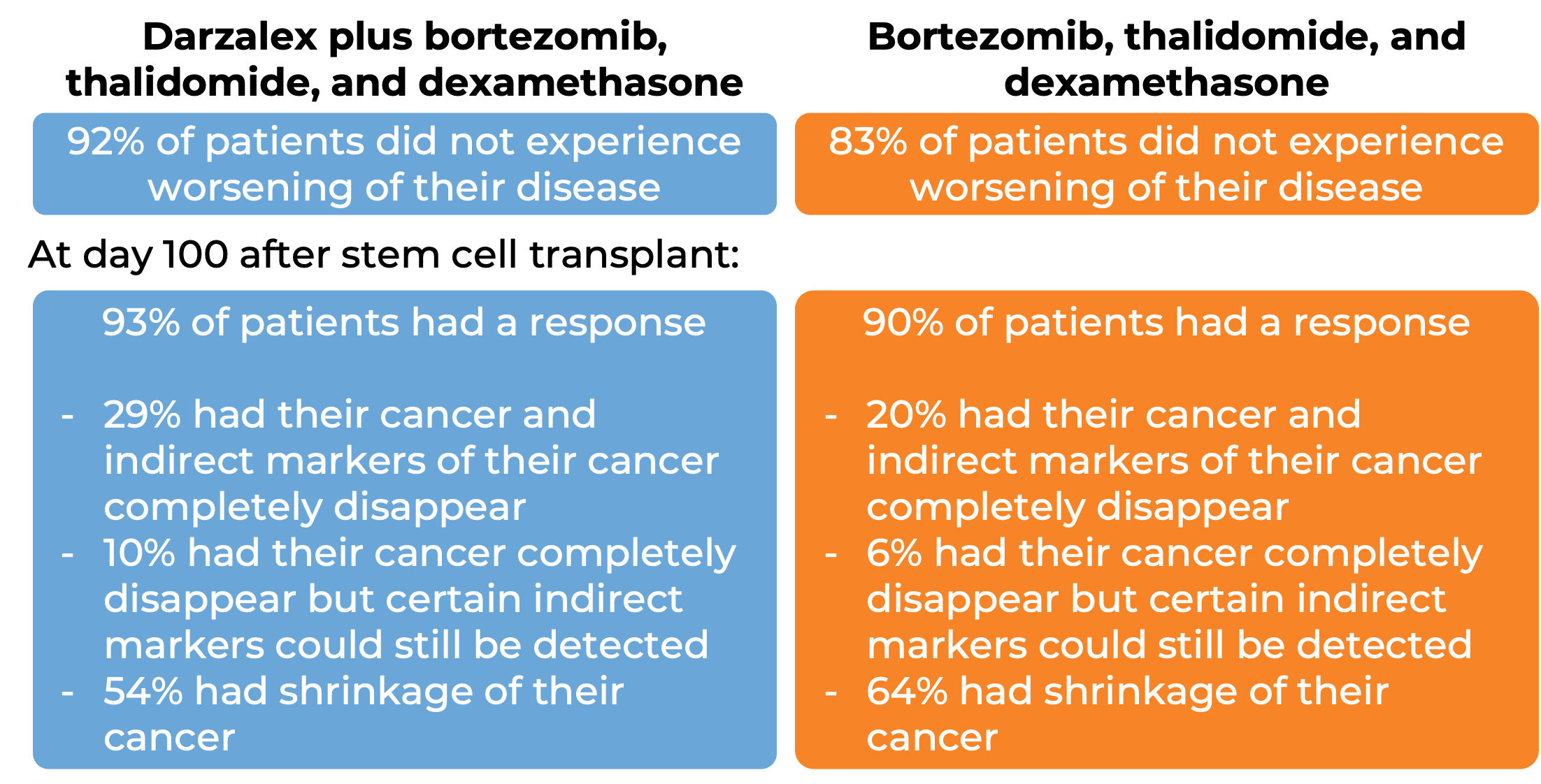
In another clinical trial, 1085 patients with newly diagnosed multiple myeloma, who could receive an autologous stem cell transplant, were treated with either:
- Darzalex, bortezomib (Velcade), thalidomide, and dexamethasone, OR
- bortezomib (Velcade), thalidomide, and dexamethasone
Patients then received high-dose chemotherapy and an autologous stem cell transplant. After the transplant, patients were again treated with either
- Darzalex, bortezomib (Velcade), thalidomide, and dexamethasone, OR
- bortezomib, thalidomide, and dexamethasone
to further improve the response to treatment and help kill any myeloma cells that may have been left in the body (consolidation therapy).
At a median follow-up of 19 months:
Multiple myeloma (previously treated)
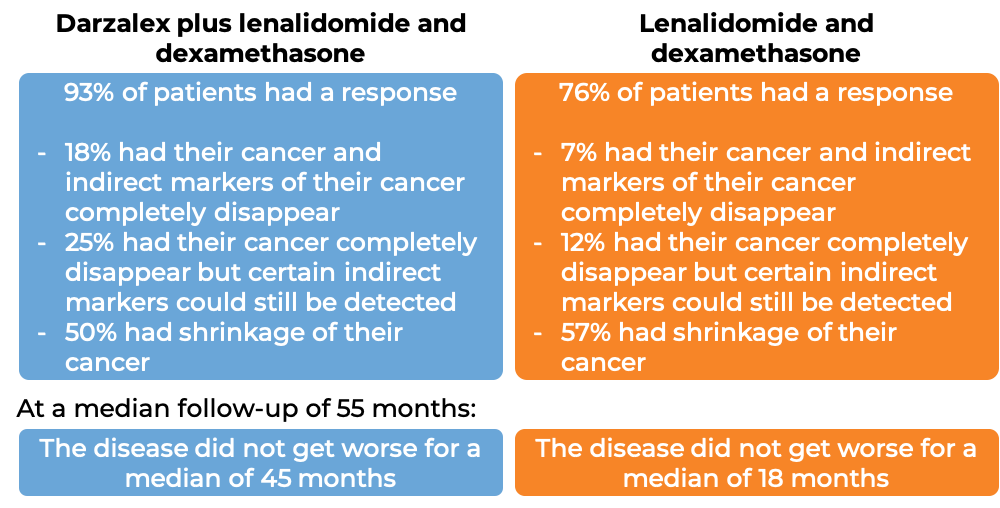
In a clinical trial, 569 patients with multiple myeloma, who had received at least one prior treatment for their disease, were treated with either:
- Darzalex, lenalidomide (Revlimid), and low-dose dexamethasone, OR
- lenalidomide and low-dose dexamethasone
At a median follow-up of 14 months:
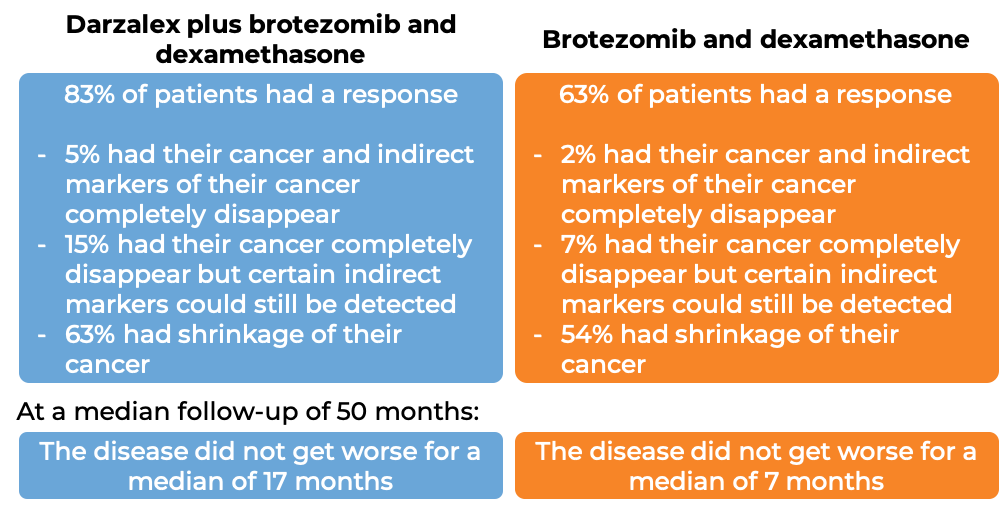
In another clinical trial, 498 patients with multiple myeloma, who had received at least one prior treatment for their disease, were treated with either:
- Darzalex, bortezomib (Velcade), and dexamethasone, OR
- bortezomib (Velcade) and dexamethasone
At a median follow-up of 7 months:
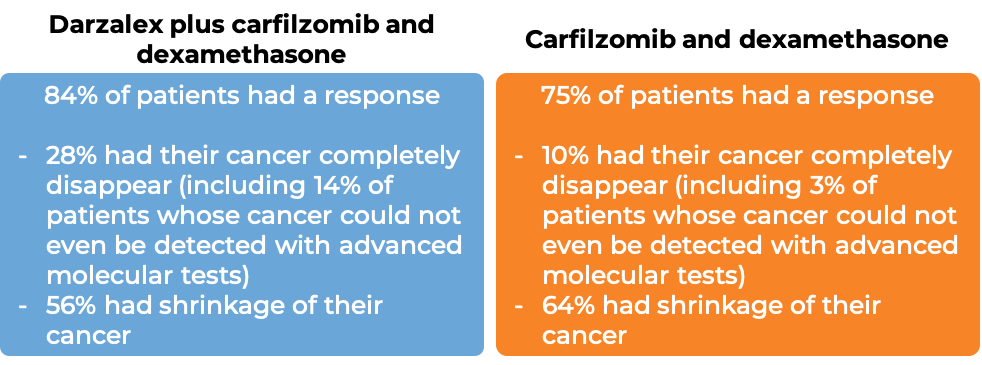
In another clinical trial, 466 patients with multiple myeloma, who had received at least 1 to 3 prior therapies for their disease and it either did not work or stopped working and the cancer had come back, were treated with either:
- Darzalex, carfilzomib (Kyprolis), and dexamethasone, OR
- carfilzomib (Kyprolis) and dexamethasone
At a median follow-up of 17 months:
In another clinical trial, 103 patients with multiple myeloma, who had received a prior proteasome inhibitor and an immunomodulatory agent for their disease, were treated with Darzalex, pomalidomide (Pomalyst), and low-dose dexamethasone.

(For the definition of “median”, click HERE).
In another clinical trial, 106 patients with multiple myeloma who had received at least three prior treatments for their disease, including a proteasome inhibitor and an immunomodulatory agent, or for whom both a proteasome inhibitor and an immunomodulatory agent did not work, were treated with Darzalex.
At a median follow-up of 15 months:

What are the side effects?
The most common side effects of Darzalex include fatigue, weakness, nausea, diarrhea, constipation, vomiting, decreased appetite, shortness of breath, cough, bronchitis, cold-like symptoms (upper respiratory infection), trouble sleeping, fever, chills, muscle spasms, back pain, joint pain, nerve damage (tingling, numbness, or pain), swelling of the hands, ankles, or feet, dizziness, lung infection (pneumonia), and low red blood cell count.
Darzalex can cause side effects that can become serious or life-threatening. Some of the serious side effects related to Darzalex include reactions related to the infusion, heart problems, pneumonia, inflammation of the airways in the lungs (bronchitis), upper respiratory tract infection, fluid buildup in the lungs, flu, low white blood cell count (which can lead to infections), and low platelet count (which can lower the ability to clot blood), and problems with the eyes.
Darzalex may interfere with the results of blood tests that are needed before a patient can receive a blood transfusion. Tests to match a patient’s blood type should be done before the first dose of Darzalex.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Janssen Biotech (US) / Janssen-Cilag International (Europe)
Approval
FDA and EMA
Links to drug websites
Other references:
- Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. Facon T, et al. The New England Journal of Medicine (2019)
- Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Mateos MV, et al. The Lancet (2020)
- Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Moreau P, et al. The Lancet (2019)
- Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. Palumbo A, et al. The New England Journal of Medicine (2016)
- Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Dimopoulos M, et al. The Lancet (2020)
Last updated: January 16, 2023
How is this drug name pronounced?
Daratumumab: DAYR-uh-TOOM-yoo-mab
Darzalex: DAR-zah-lex
What cancer(s) does this drug treat?
Multiple myeloma (newly diagnosed)
Darzalex is approved for:
- Patients with newly diagnosed multiple myeloma who cannot receive an autologous stem cell transplant (stem cell transplant that uses their own stem cells). In such cases, Darzalex is used in combination with lenalidomide (Revlimid) and dexamethasone, OR in combination with bortezomib (Velcade), melphalan, and prednisone.
- Patients with newly diagnosed multiple myeloma who can receive an autologous stem cell transplant. In such cases, Darzalex is used in combination with bortezomib (Velcade), thalidomide, and dexamethasone.
Multiple myeloma (previously treated)
Darzalex is approved for:
- Patients with multiple myeloma who have received at least one prior treatment for their disease. In such cases, Darzalex is used in combination with lenalidomide (Revlimid) and dexamethasone, OR in combination with bortezomib (Velcade) and dexamethasone.
- Patients with multiple myeloma who have received at least one prior treatment containing lenalidomide (Revlimid) and a proteasome inhibitor, and it did not work. In such cases, Darzalex is used in combination with pomalidomide (Pomalyst) and dexamethasone.
- Patients with multiple myeloma who have received prior treatment for their disease, including a proteasome inhibitor and an immunomodulatory agent, and it either did not work (refractory disease) or stopped working (relapsed disease). In such cases, Darzalex is used by itself.
Limitations of use:
Age: The safety and efficacy of Darzalex in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: The risks associated with Darzalex during pregnancy are not known and cannot be ruled out. Due to the potential for harm to the fetus, Darzalex is not recommended for use during pregnancy. Women are advised to use contraception during treatment with Darzalex and for at least 3 months after the last dose of Darzalex. The risks associated with Darzalex during breastfeeding are not known and cannot be ruled out. Due to the potential for adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Darzalex.
Body weight: Darzalex for subcutaneous use may be less effective in patients who weigh over 120 kg.
Limitations: Darzalex contains sorbitol and should not be administered to patients who are hereditarily fructose intolerant.
What type of immunotherapy is this?
How does this drug work?
Darzalex is an antibody that was made in the laboratory. Darzalex and other antibody molecules have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. Darzalex attaches to a molecule called CD38. CD38 plays a role in the attachment of neighboring cells to each other, and in transmitting messages from outside of the cell to the inside of the cell. CD38 is present on the surface of many types of immune cells (white blood cells) and red blood cells, and is also found in high amounts on the surface of myeloma cells.
The stem of Darzalex’s “Y” shape has binding sites for immune cells or other parts of the immune system.
At least five different mechanisms are thought to be responsible for the elimination of myeloma cells by Darzalex. Darzalex works alone and/or is helped by the immune system to kill myeloma cells.
Direct cell killing
By binding to CD38 molecules on the surface of myeloma cells and bringing the CD38 molecules close together in “clusters”, Darzalex can directly cause the cells to die.
Complement-dependent cytotoxicity (CDC)
When bound to CD38 on the surface of myeloma cells, the “stem” of Darzalex can attract and bind molecules of the immune system called “complement” that freely flow in the blood or tissues. Activation of the complement system causes the formation of the “membrane attack complex”, which can puncture and destroy the cell that Darzalex is bound to.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
When bound to CD38 on the surface of myeloma cells, the “stem” of Darzalex can also attract and bind immune cells. This allows Darzalex to act as a bridge between the target cell and the immune cell (such as the natural killer (NK) cell). The immune cell then releases molecules that can kill the cell that Darzalex is bound to.
Phagocytosis
When Darzalex is bound to myeloma cells, it can also attract immune cells called phagocytes, which have the ability to ingest (“eat”) cells that have been coated with Darzalex.
Strengthening the immune response
Darzalex can bind to CD38 on the surface of certain types of immunosuppressive immune cells that reduce the strength of an immune response, causing such cells to die. At the same time Darzalex can activate other immune cells that are required for the elimination of cancer cells, thus allowing for a stronger immune attack on myeloma cells.
The combined effect of these mechanisms results in the elimination of melanoma from the body. Because CD38 is also found on certain normal cells, Darzalex can attack and reduce the numbers of some types of immune cells, including healthy B cells and natural killer (NK) cells.

How is this drug given to the patient?
About 1 to 3 hours prior to receiving each dose of Darzalex, patients receive several medications to help reduce the chance of a reaction to the infusion:
- A corticosteroid (dexamethasone or methylprednisolone)
- An antihistamine (diphenhydramine)
- A painkiller and fever reducer (paracetamol)
Darzalex is available in two different forms: one is for administration via a tube into a vein (intravenous [i.v.] infusion) and the other is for administration via an injection under the skin (subcutaneous [s.c.] injection) of the abdomen. Darzalex is given every one, two, three, or four weeks, depending on the treatment regimen and how long the patient has been receiving Darzalex. The first infusion of Darzalex for intravenous use may take, on average, about 7 hours, although the first dose may be split between two shorter infusions over two consecutive days. Subsequent infusions typically take 3 to 5 hours to administer. Each injection of Darzalex for subcutaneous use is given over 3-5 minutes. Darzalex for subcutaneous use additionally contains hyaluronidase, which can increase the ability ofDarzalex to infiltrate into tissue under the skin and enter the bloodstream.
In most cases, Darzalex is administered in combination with other medications. In such cases, a corticosteroid may be part of the treatment regimen, in addition to the corticosteroid that is given before Darzalex administration.
Patients may receive corticosteroids after each administration of Darzalex to reduce the risk of a delayed reaction to the infusion.
Patients may receive antiviral medication to prevent herpes zoster (shingles) reactivation.
What are the observed clinical results?
For:
Multiple myeloma (newly diagnosed)
Multiple myeloma (previously treated)
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Multiple myeloma (newly diagnosed)
Darzalex in combination with lenalidomide (Revlimid) and low-dose dexamethasone
In a clinical trial, 737 patients with newly diagnosed multiple myeloma, who could not receive an autologous stem cell transplant (stem cell transplant that uses their own stem cells), were treated with either:
- Darzalex for intravenous use, lenalidomide (Revlimid), and low-dose dexamethasone, OR
- lenalidomide and low-dose dexamethasone
At a median follow-up of 28 months:
Darzalex in combination with bortezomib (Velcade), melphalan, and prednisone (D-VMP)
In another clinical trial, 706 patients with newly diagnosed multiple myeloma, who could not receive an autologous stem cell transplant, were treated with either:
- Darzalex for intravenous use, bortezomib (Velcade), melphalan, and prednisone, OR
- bortezomib (Velcade), melphalan, and prednisone
At a median follow-up of 17 months:

In another clinical trial, 67 patients with newly diagnosed multiple myeloma who could not receive an autologous stem cell transplant, were treated with Darzalex for subcutaneous use plus bortezomib (Velcade), melphalan, and prednisone. At a median follow-up of 14 months, 90% of patients saw their cancer shrink in response to the treatment, with 48% seeing their cancer disappear entirely.
Darzalex in combination with bortezomib (Velcade), thalidomide, and prednisone
In another clinical trial, 1085 patients with newly diagnosed multiple myeloma, who were eligible to receive an autologous stem cell transplant, were treated with either:
- Darzalex for intravenous use, bortezomib (Velcade), thalidomide, and dexamethasone, OR
- bortezomib (Velcade), thalidomide, and dexamethasone
Patients then received high-dose chemotherapy and an autologous stem cell transplant. After the transplant, the patients were again treated with either
- Darzalex for intravenous use, bortezomib (Velcade), thalidomide, and dexamethasone, OR
- bortezomib (Velcade), thalidomide, and dexamethasone
to further improve the response to treatment and help kill any myeloma cells that may have been left in the body (consolidation therapy).
At a median follow-up of 19 months:
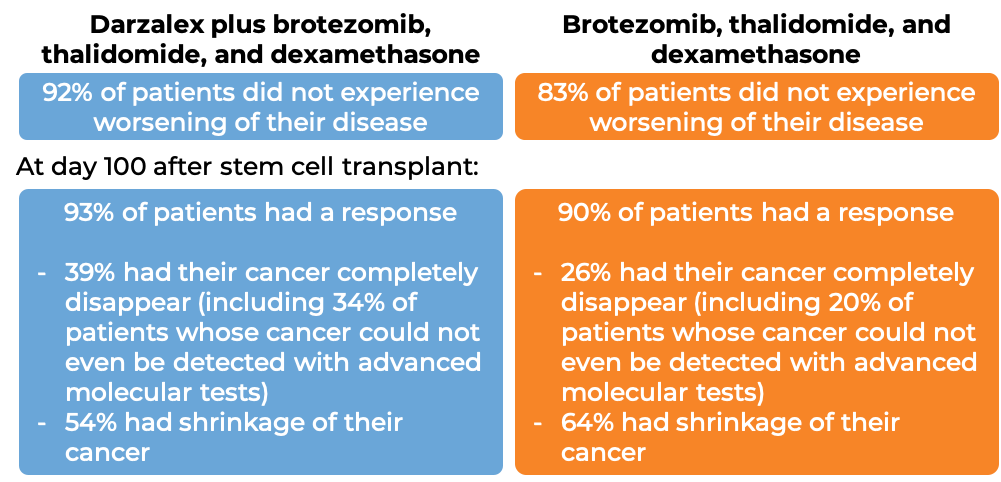
Multiple myeloma (previously treated)
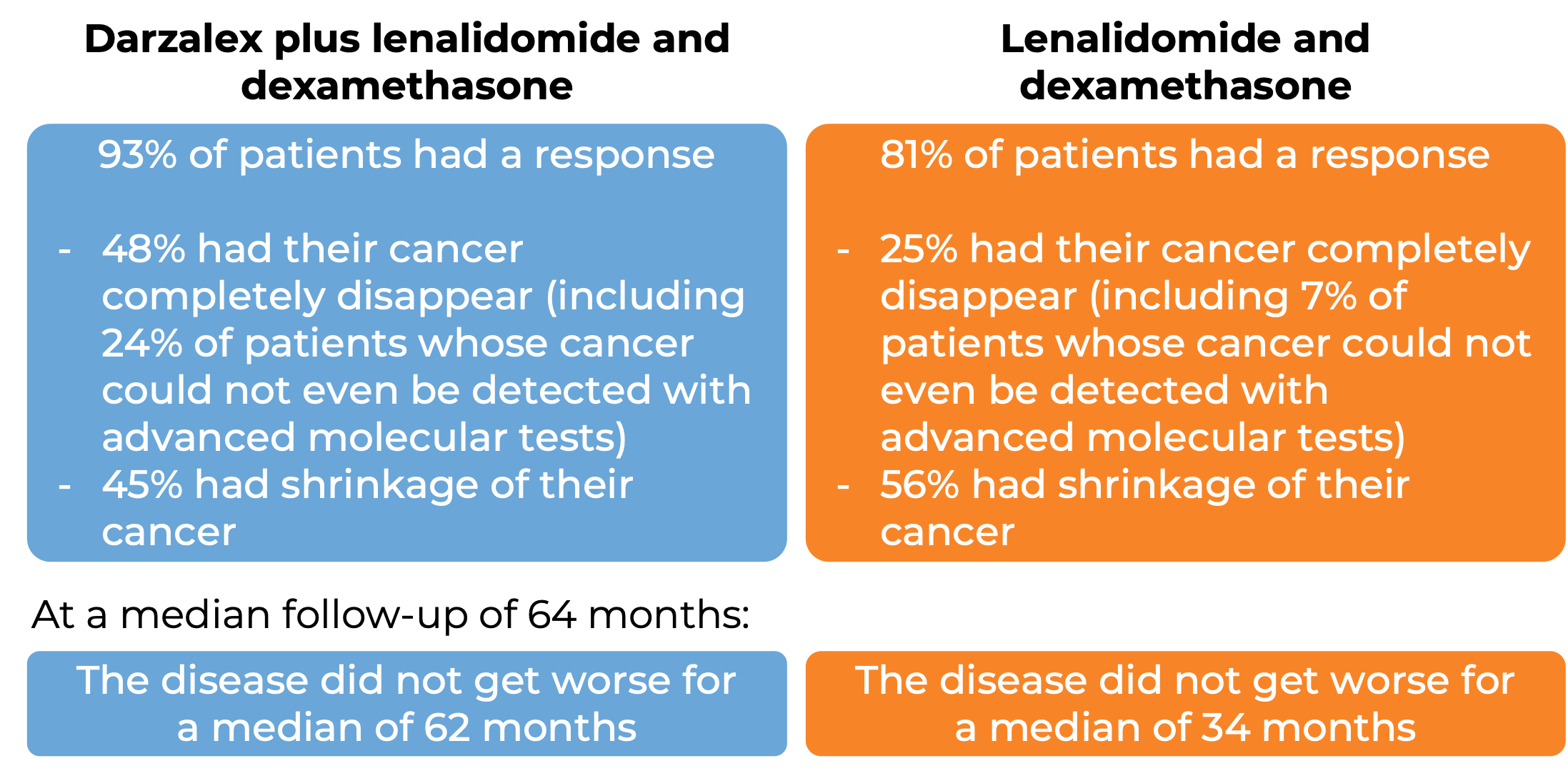
Darzalex in combination with lenalidomide (Revlimid) and low-dose dexamethasone (D-Rd)
In a clinical trial, 569 patients with multiple myeloma, who had received at least one prior treatment for their disease and it either did not work or stopped working and the cancer came back, were treated with:
- Darzalex for intravenous use, lenalidomide (Revlimid), and low-dose dexamethasone, OR
- lenalidomide and low-dose dexamethasone
At a median follow-up of 14 months:

At a longer-term median follow-up of 80 months, patients treated with Darzalex plus lenalidomide and dexamethasone survived for a median of 68 months, while those treated with lenalidomide and dexamethasone alone survived for a median of 52 months.
In another clinical trial, 65 patients with multiple myeloma whose cancer had been previously treated, but it either did not respond to treatment (refractory) or had since returned (relapsed), were treated with Darzalex for subcutaneous use plus lenalidomide (Revlimid) and dexamethasone. At a median follow-up of 15 months, 94% of patients saw their cancer shrink in response to the treatment, with 39% seeing their cancer disappear entirely.
Darzalex in combination with bortezomib (Velcade) and dexamethasone
In a clinical trial, 498 patients with multiple myeloma, who had received at least one prior treatment for their disease and it either did not work or stopped working and the cancer came back, were treated with:
- Darzalex for intravenous use, bortezomib (Velcade), and dexamethasone, OR
- bortezomib (Velcade) and dexamethasone
At a median follow-up of 7 months:

At a longer-term median follow-up of 73 months, patients treated with Darzalex plus bortezomib and dexamethasone survived for a median of 50 months, while those treated with bortezomib and dexamethasone alone survived a median of 39 months.
Darzalex in combination with pomalidomide (Pomalyst) and dexamethasone
In a clinical trial, 304 patients with multiple myeloma who had received at least one prior treatment containing lenalidomide (Revlimid) and a proteasome inhibitor, were treated with Darzalex for subcutaneous use in combination with pomalidomide (Pomalyst) and dexamethasone OR with pomalidomide and dexamethasone alone. At a median follow-up of 17 months:
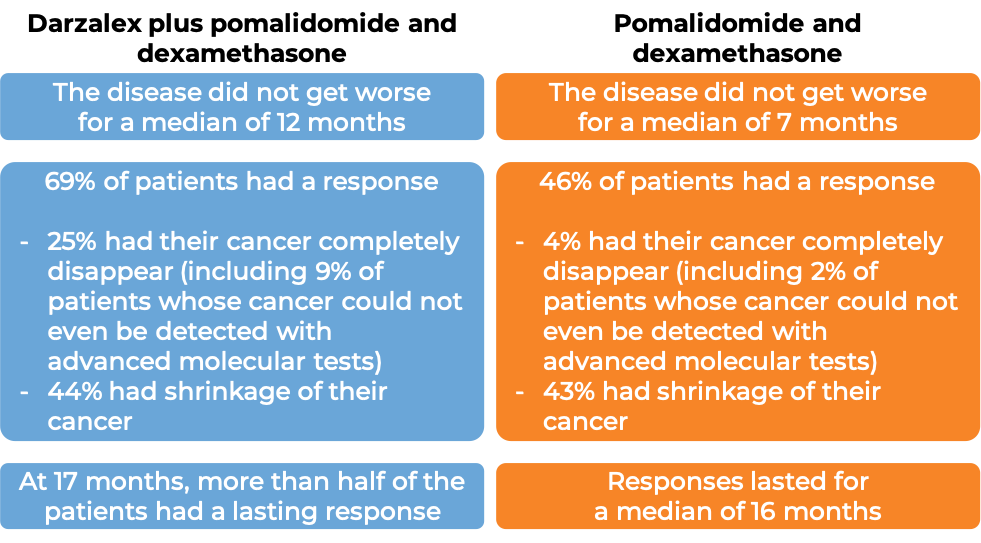
At a longer-term median follow-up of 40 months, patients treated with Darzalex plus pomalidomide and dexamethasone survived for a median of 34 months, while those treated with pomalidomide and dexamethasone alone survived for a median of 24 months.
Darzalex used by itself In a clinical trial, 106 patients with multiple myeloma who had received prior treatment for their disease, including a proteasome inhibitor and an immunomodulatory agent, and it either did not work or stopped working, and the cancer came back, were treated with Darzalex for intravenous use. At a median follow-up of 15 months:

In another clinical trial, 522 patients with multiple myeloma who had received at least 3 prior treatments, including a proteasome inhibitor and an immunomodulatory agent, but whose cancer either did not respond to treatment (refractory) or had returned since treatment (relapsed), were randomized to receive either Darzalex for subcutaneous use or Darzalex for intravenous use. This trial was set out to study whether both Darzalex formulations would deliver a similar benefit to patients. At a median follow-up of 8 months:
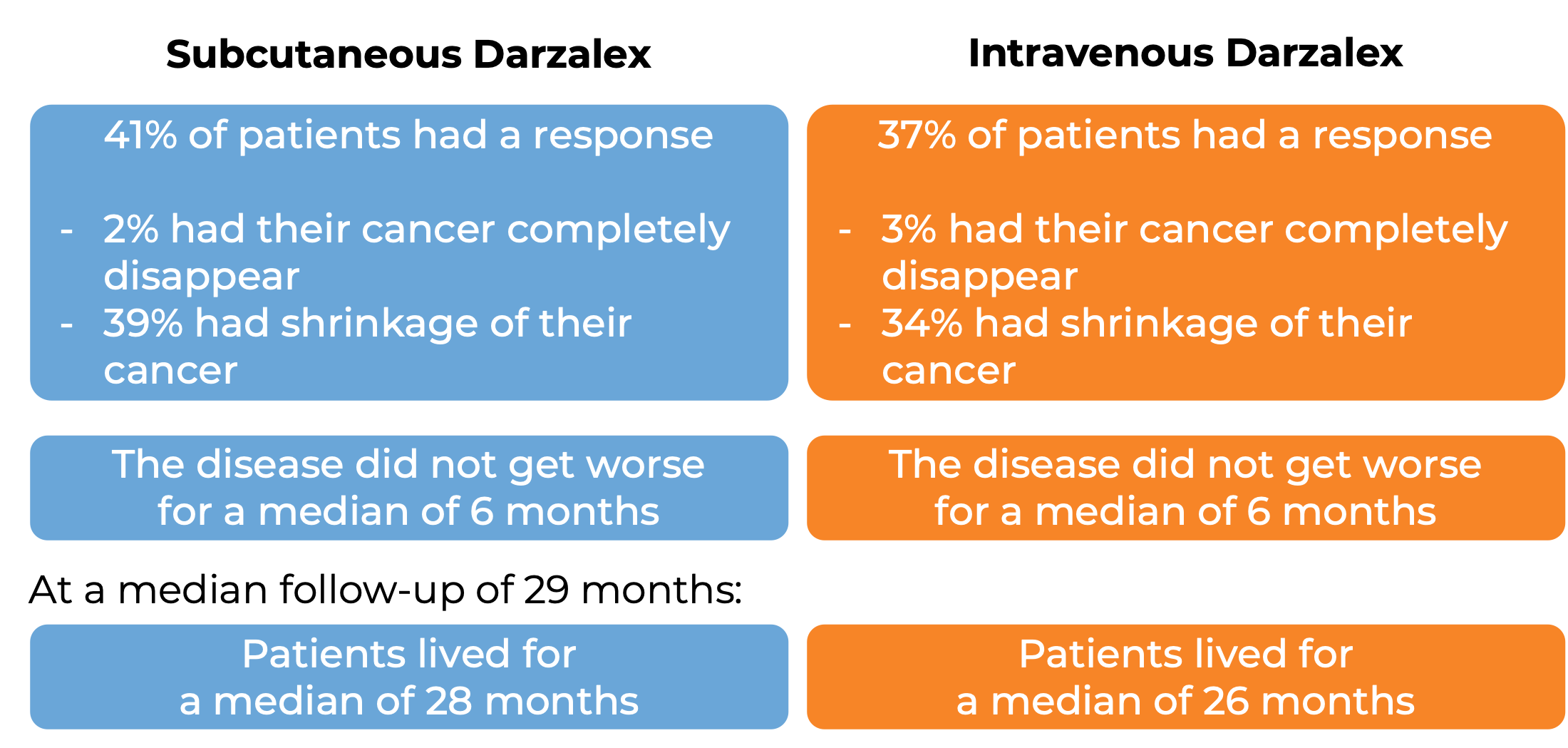
What are the side effects?
The most common side effects of Darzalex include fatigue, weakness, nausea, diarrhea, constipation, vomiting, decreased appetite, shortness of breath, cough, bronchitis, cold-like symptoms (upper respiratory infection), trouble sleeping, fever, chills, muscle spasms, back pain, joint pain, nerve damage (tingling, numbness, or pain), swelling of the hands, ankles, or feet, dizziness, lung infection (pneumonia), and low red blood cell count.
Darzalex can cause side effects that can become serious or life-threatening. Some of the serious side effects related to Darzalex include reactions related to the infusion or injection, heart problems, pneumonia, inflammation of the airways in the lungs (bronchitis), upper respiratory tract infection, fluid buildup in the lungs, flu, low white blood cell count (which can lead to infections), and low platelet count (which can lower the ability to clot blood).
Darzalex may interfere with the results of blood tests that are needed before a patient can receive a blood transfusion. Tests to match a patient’s blood type should be done before the first dose of Darzalex.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Janssen Biotech (US) / Janssen-Cilag International (Europe)
Approval
FDA and EMA
Links to drug websites
Other references:
Last updated: July 16, 2024















