How is the drug name pronounced?
Tagraxofusp: Tuh-GRAX-uh-fusp
Elzonris: El-ZON-riss
What cancer(s) does this drug treat?
Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN)
Elzonris is approved for:
- Adult and pediatric patients 2 years and older with blastic plasmacytoid dendritic cell neoplasm.
Limitations of Use
Age: The safety and efficacy of Elzonris has not been established in patients under 2 years of age.
Pregnancy/breastfeeding: Elzonris may cause harm to the fetus and is not recommended for use during pregnancy. Women should not start Elzonris treatment when pregnant and are advised to use contraception during treatment with Elzonris and for at least one week after the last dose of Elzonris. The risks associated with Elzonris during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Elzonris and for at least one week after the last dose of Elzonris.
What type of immunotherapy is this?
- Cytotoxin
How does this drug work?
Target:
- Interleukin-3 receptor (CD123)
Elzonris is a medicine that was made in the laboratory and consists of two parts that are connected: a small molecule, called interleukin-3, and a cell poison, called diphtheria toxin, that was obtained from a bacterium. The interleukin-3 portion of the Elzonris attaches to another molecule, known as interleukin-3 receptor (CD123), on the surface of BPDCN cells. When Elzonris attaches to the interleukin-3 receptor, it can enter BPDCN cells. Inside the cell, the diphtheria toxin part of Elzonris is released, preventing the cell from making essential proteins and leading to cell death.
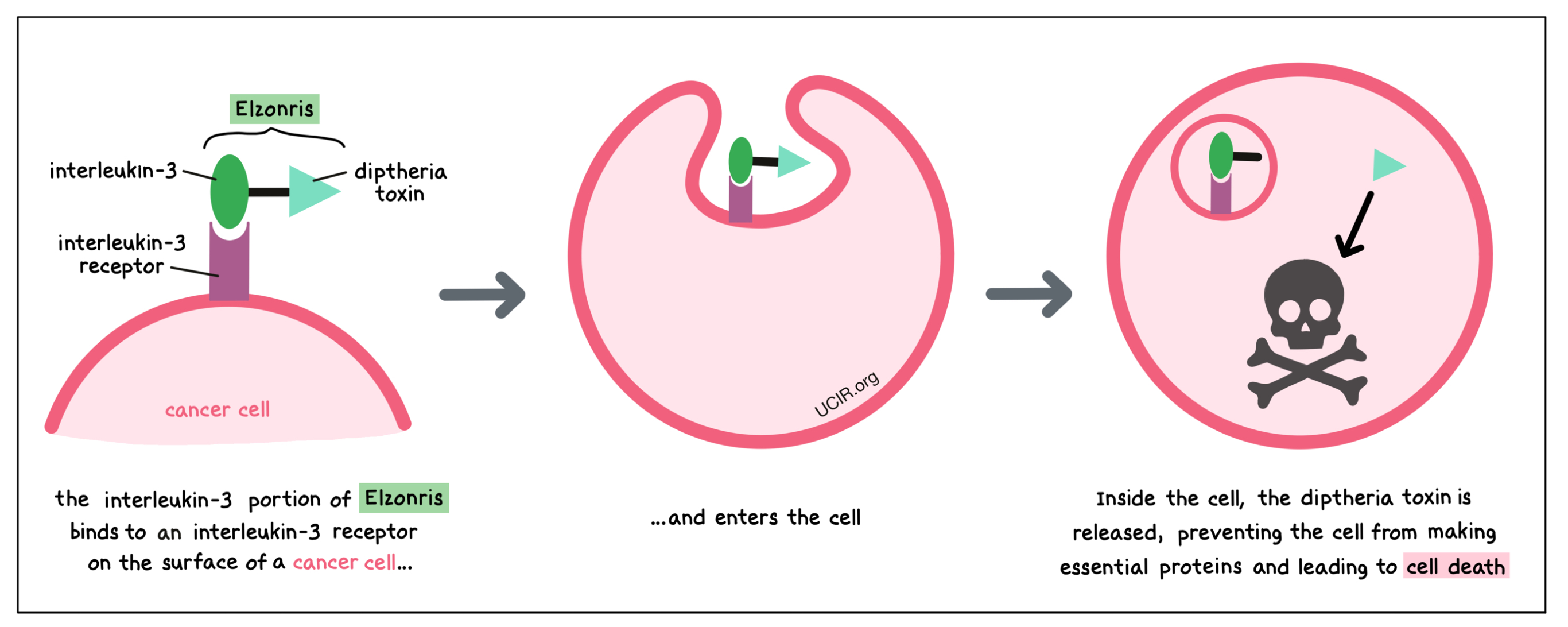
The interleukin-3 receptor (CD123) is present on the surface of some blood-forming cells and some dendritic cells. In a healthy person, it regulates the cells’ ability to multiply and to become more specialized. In BPDCN, the cancerous cells have high levels of the interleukin-3 receptor on their surface. By targeting the interleukin-3 receptor, Elzonris is designed to minimize harm to normal, healthy cells and to bring the cell poison directly to the cancer cells.
How is this drug given to the patient?
Before starting Elzonris therapy, patients will have their vital signs checked and will need to get blood tests done to monitor liver function.
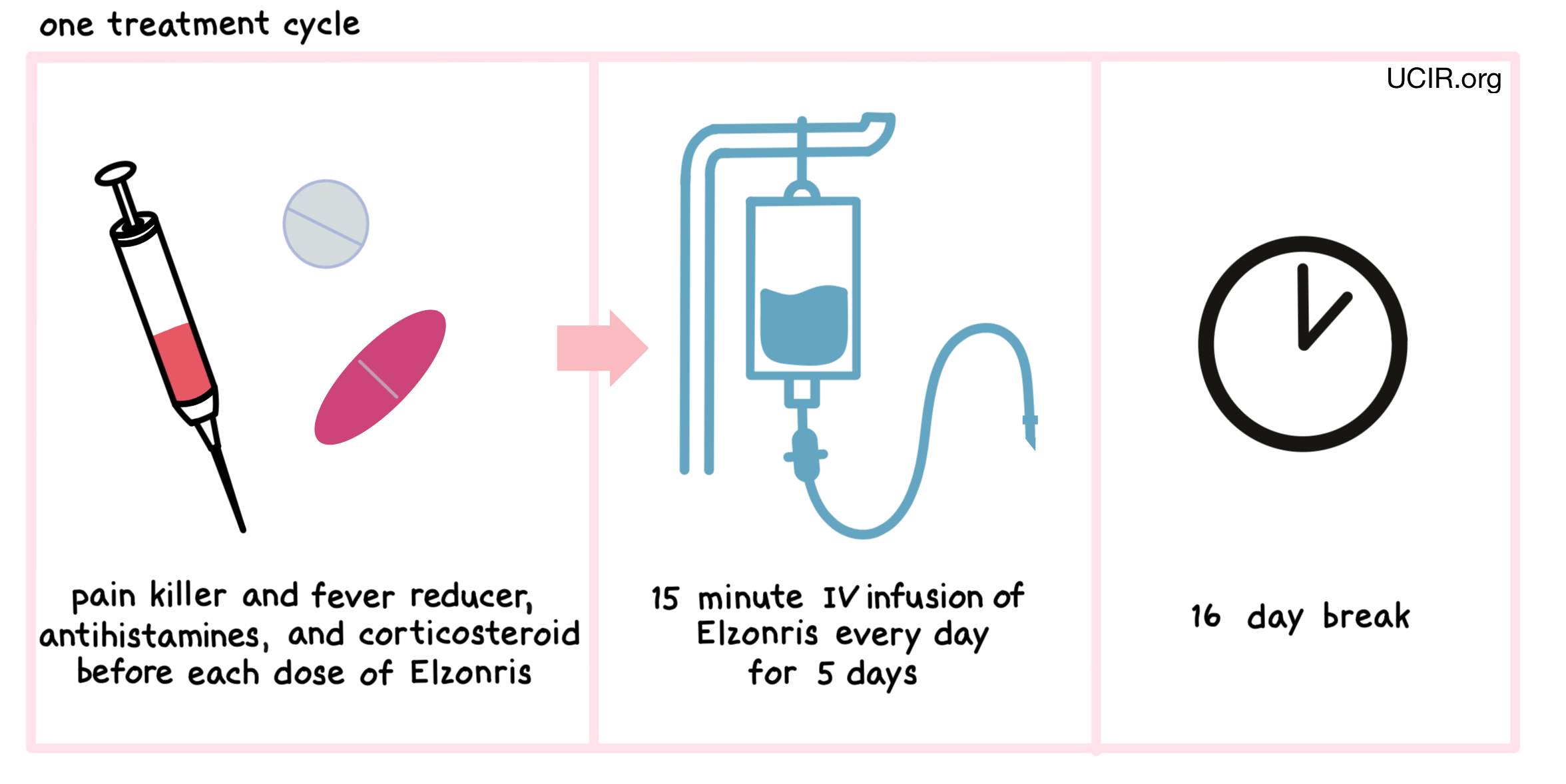
One hour before to each administration of Elzonris, patients receive several medications to help reduce the chance of a reaction to the infusion:
- A corticosteroid (e.g. methylprednisolone via a tune in the vein)
- An H1 antihistamine (e.g. diphenhydramine hydrochloride)
- An H2 antihistamine (e.g. famotidine)
- A painkiller and fever reducer (e.g. acetaminophen)
Elzonris is administered via a tube in the vein (intravenous infusion, or I.V.) over 15 minutes once daily on days 1 through 5 of a 21 day treatment period. Each 21 day treatment period is one treatment “cycle”. If a patient shows symptoms of any serious side effects, treatment may be interrupted or temporarily withheld until the side effects subside. In the event of such a dose delay, the dose period may be extended up to day 10 of a treatment cycle. During the first treatment cycle, patients are required to stay in a hospital. Subsequent treatment cycles will be monitored by a healthcare professional during and for at least four hours after each dose, and may not require a hospital stay.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies.
Previously untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN)
In a clinical trial, 13 patients with previously untreated blastic plasmacytoid dendritic cell neoplasm were treated with Elzonris. At a median follow-up of 12 months, 7 patients (54%) had their cancer completely disappear within 14 to 107 days. Some of these 7 patients were cancer-free, but still had abnormal parts of their skin. Some patients went on to receive a stem cell transplant.
Previously treated blastic plasmacytoid dendritic cell neoplasm (BPDCN)
In a clinical trial, 15 patients with blastic plasmacytoid dendritic cell neoplasm who had previously received treatment for their disease, but whose cancer either did not respond to the treatment (refractory) or had since returned (relapsed), were treated with Elzonris. Of the 15 patients, 10 (67%) patients responded to Eronris treatment within 17 to 48 days, and responses lasted for a median of 3 months. More than half of the 15 patients were alive after 9 months. One patient went on to receive a stem cell transplant.
What are the potential side effects?
The most common side effects of Elzonris include fatigue, headache, nausea, swelling in lower legs and arms, weight gain, back pain, low or high blood pressure, vomiting, constipation, diarrhea, elevated heart rate, fever, chills, coughing, difficulty breathing, decreased appetite, and abnormal blood test results.
Elzonris can cause side effects that can become serious or life-threatening. Some of the serious side effects related to Elzonris include Capillary Leak Syndrome, hypersensitivity reactions, liver damage, and tumor lysis syndrome. Patients should immediately report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
Capillary Leak Syndrome
Elzonris may cause fluid to leak from small blood vessels into surrounding tissues, which is a serious, potentially life-threatening condition that can result in low blood pressure and fast weight gain. Other symptoms of Capillary Leak Syndrome include weakness or dizziness, tiredness, swelling of the face, arms, or legs, and shortness of breath or trouble breathing.
Hypersensitivity reactions
Hypersensitivity reactions are allergic reactions caused by the body’s immune system responding to Elzonris as if it were a threat. Symptoms of a hypersensitivity reaction may include rashes, itching, wheezing, swelling of the face, shortness of breath, and swelling of the airways.
Liver damage
Elzonris is known to have the potential to cause damage to the liver. Symptoms of liver damage may include abnormal measures of liver function in the blood, fatigue, loss of appetite, yellowing of the skin or the whites of the eyes, and upper right abdominal pain.
Tumor lysis syndrome
Tumor lysis syndrome is caused by the rapid breakdown of cancer cells. TLS can cause kidney failure. Symptoms of TLS include weakness, shortness of breath, feeling confused, irregular heartbeat, and muscle cramps.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer
Stemline Therapeutics
Approval
FDA and EMA
Website
Other references
Last updated on April 2, 2024


