How is this drug name pronounced?
Durvalumab: dur-VAL-yoo-mab
Imfinzi: im-FIN-zee
What cancer(s) does this drug treat?
Imfinzi has been approved for a number of cancer types and stages, in some cases as a single therapy and in some cases in combination with other therapies.
Imfinzi is approved for:
Non-small cell lung cancer
Small cell lung cancer
Biliary tract cancer
Liver cancer
Endometrial cancer
Non-small cell lung cancer
Imfinzi is approved for:
-
Patients with non-small cell lung cancer whose cancer
In such cases, Imfinzi is given in combination with platinum-containing chemotherapy prior to surgery and is continued after surgery to prevent the cancer from coming back.
-
Patients with non-small cell lung cancer whose cancer:
- has not spread outside the chest (Stage III), AND
- cannot be removed by surgery, AND
- has responded to or stabilized after treatment with chemotherapy containing platinum given at the same time as radiation therapy.
In such cases, Imfinzi is given alone.
- Patients with non-small cell lung cancer that has spread to other parts of the body and does not have an abnormal EGFR or ALK gene. In such cases, Imfinzi is used in combination with platinum-based chemotherapy and tremelimumab (Imjudo).
Advanced small cell lung cancer
Imfinzi is approved for:
- Patients with extensive-stage small cell lung cancer (cancer that has spread to other parts of the body). In such cases, Imfinzi is used in combination with etoposide (Etopophos, Toposar) and either carboplatin (Paraplatin) or cisplatin, as a first treatment.
Biliary tract cancer
Infinzi is approved for:
- Patients with biliary tract cancer (including bile duct cancer/cholangiocarcinoma and gallbladder cancer) that has spread to nearby lymph nodes or organs (locally advanced) and/or to other more distant parts of the body (metastatic). In such cases, Imfinzi is used in combination with gemcitabine and cisplatin chemotherapy.
Advanced liver cancer
Imfinzi is approved for:
- Patients with liver cancer (hepatocellular carcinoma) that cannot be removed by surgery. In such cases, Imfinzi is used in combination with tremelimumab (Imjudo).
Endometrial cancer
Imfinizi is approved for:
- Patients with endometrial cancer that is mismatch repair-deficient (dMMR) and is advanced or has come back (recurrent). In such cases, Imfinzi is used first in combination with carboplatin and paclitaxel and then on its own.
Limitations of use
Age: The safety and efficacy of Imfinzi in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Imfinzi can cause harm to a fetus and is not recommended for use during pregnancy. Pregnancy should be prevented for at least three months after the last dose of Imfinzi. The risks associated with Imfinzi during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, patients are advised not to breastfeed during treatment and for at least three months after the last dose of Imfinzi. Patients should also refer to the prescribing information of agents that are given in combination with Imfinzi for additional recommendations.
Complications of stem cell transplant: Serious and life-threatening complications, that can lead to death, can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Imfinzi.
Solid organ transplant rejection: Treatment with Imfinzi can increase the risk of rejection in patients who have received an organ transplant or other transplant (including corneal graft).
What type of immunotherapy is this?
- PD-L1 blockade
How does this drug work?
- Target: PD-L1
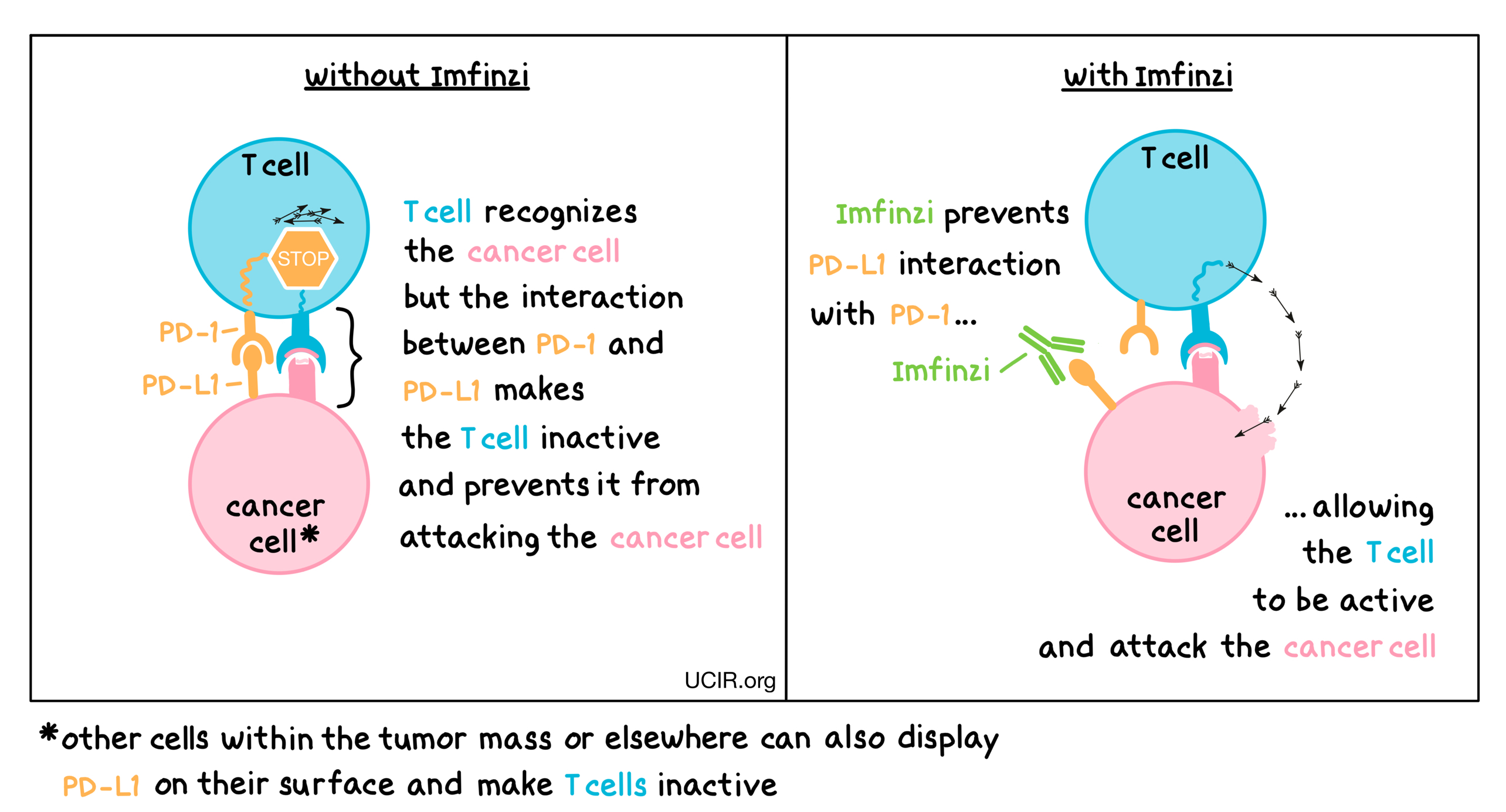
Imfinzi is an antibody that attaches to a molecule called PD-L1, which is sometimes present on the surface of cancer cells or other cells within a tumor mass. PD-L1 interacts with a molecule called PD-1, which is present on the surface of T cells – the primary immune cells involved in killing cancer cells. In healthy tissues, the interaction between PD-L1 and PD-1 puts on a brake that keeps T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from doing their job – killing the cancer cells. When PD-L1 interacts with PD-1 on T cells, the T cells become inactive and do not attack the cancer cells. Imfinzi binds to the PD-L1 molecules on cancer cells and other cells within a tumor mass in such a way that it blocks the interaction between PD-1 and PD-L1, and allows the T cells to be active and attack the cancer cells.
How is this drug given to the patient?
Imfinzi is administered via a tube into a vein (intravenous infusion, or i.v.) over 60 minutes every 2, 3, or 4 weeks, depending on the cancer type and other factors, and does not require a hospital stay. When given in combination with chemotherapy, Imfinzi is administered prior to chemotherapy on the same day.
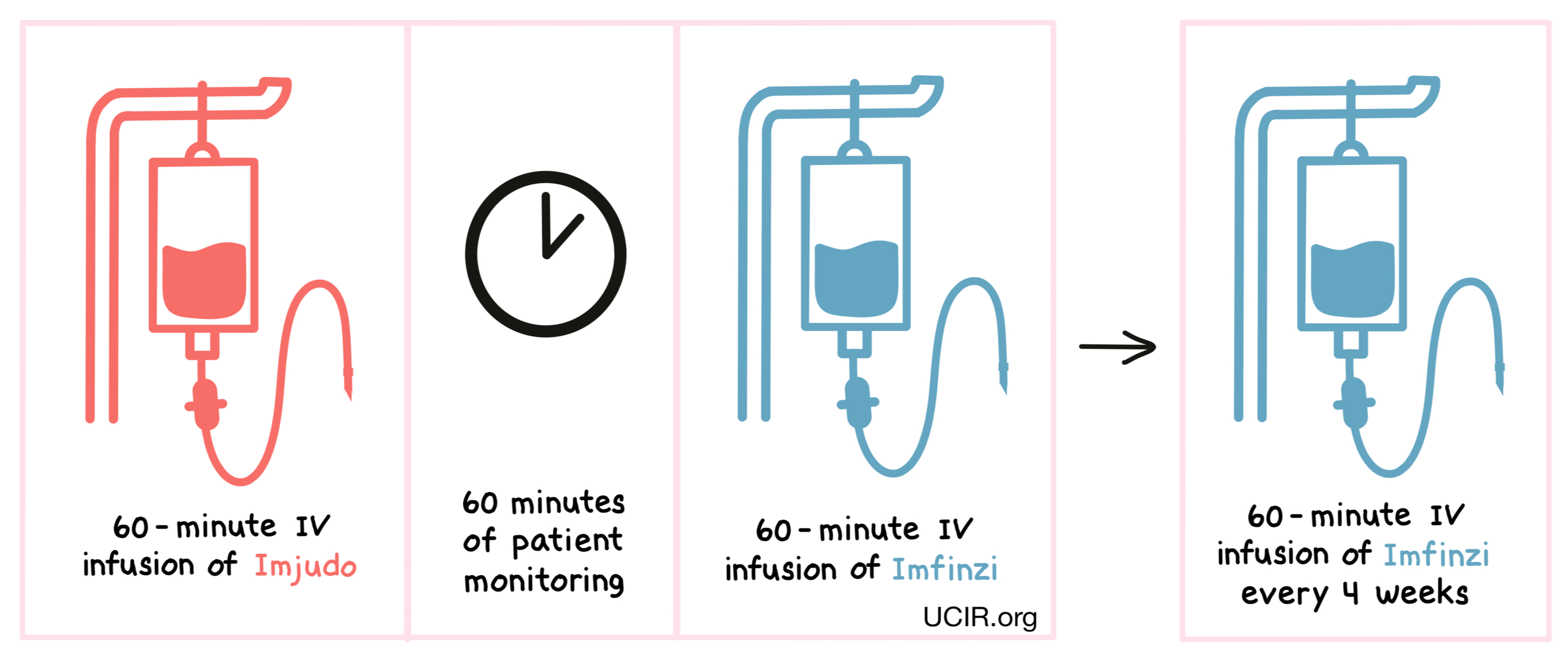
For patients with liver cancer, tremelimumab (Imjudo) is administered via a tube into a vein (i.v.) over 60 minutes. Patients are then monitored for 60 minutes before receiving Imfinzi via a tube in the vein over 60 minutes. Therapy is then continued with only Imfinzi, administered every 4 weeks.
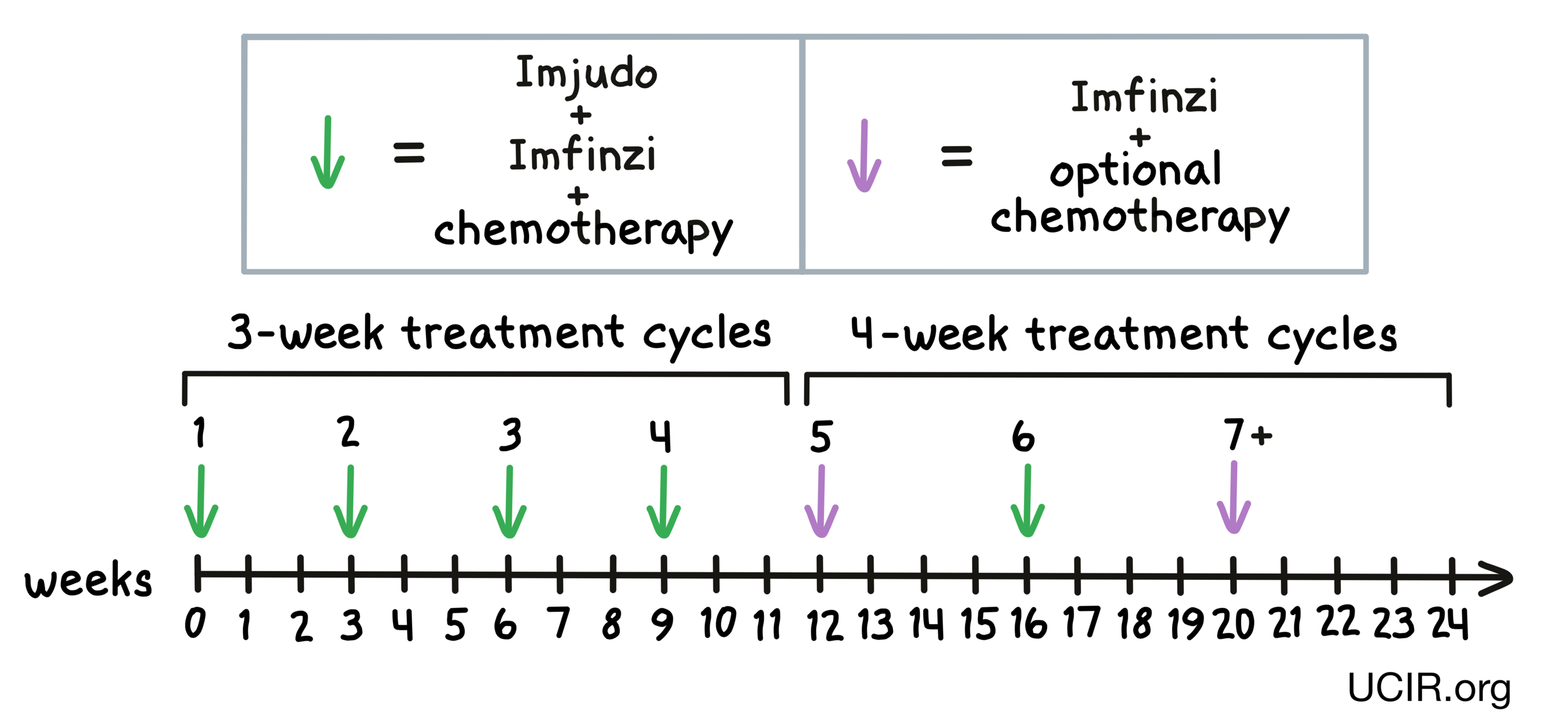
For patients with metastatic non-small cell lung cancer (cancer that has spread to other parts of the body, Imfinzi, tremelimumab (Imjudo), and platinum-based chemotherapy are given together (on the same day) at the beginning of each treatment cycle for treatment cycles 1 to 4 and treatment cycle 6. For the fifth treatment cycle, and for treatment cycles 7 and on, patients are treated only with Imfinzi and optional chemotherapy. Treatment cycles 1 through 4 are each 3 weeks long, while treatment cycles 5 and on are 4 weeks long.
What are the observed clinical results?
For:
Advanced non-small cell lung cancer
Advanced small cell lung cancer
Biliary tract cancer
Advanced liver cancer
Endometrial cancer
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Non-small cell lung cancer
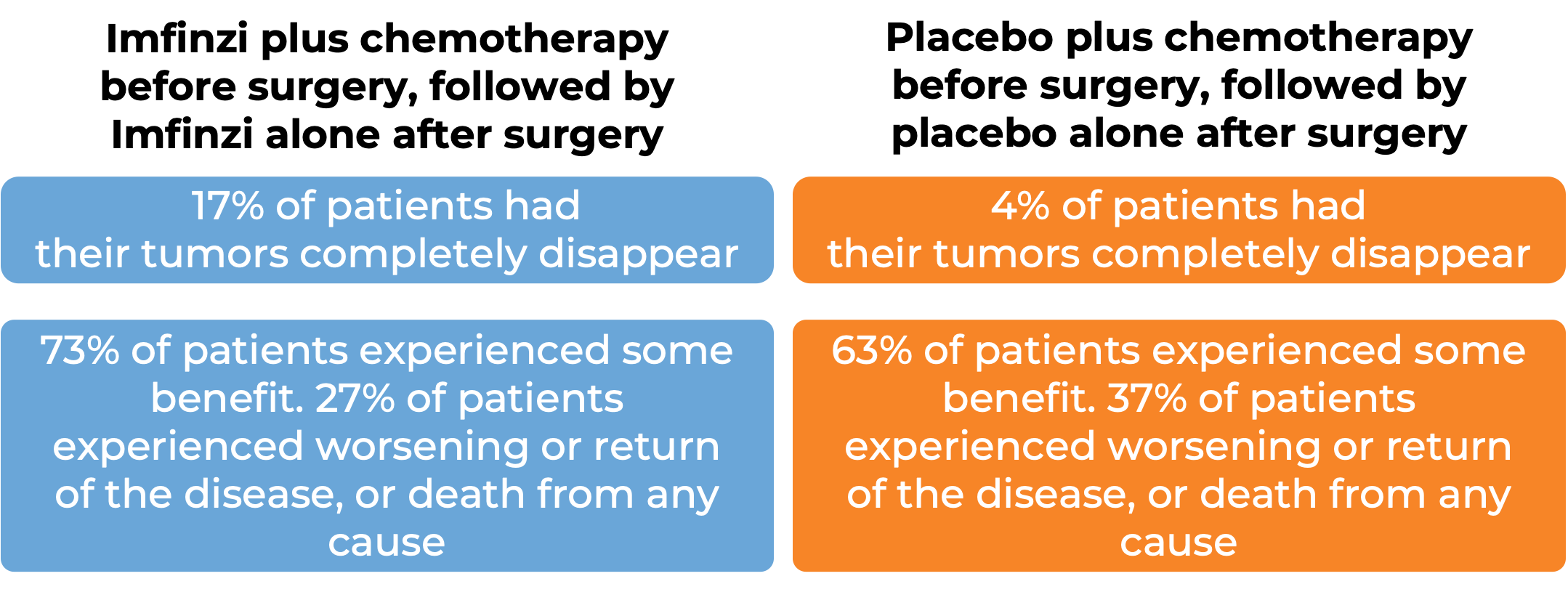
In a clinical trial, 799 patients with non-small cell lung cancer whose cancer could be removed by surgery and who did not have an abnormal EGFR or ALK gene were treated with either:
- Imfinzi plus platinum-containing chemotherapy prior to surgery followed by Imfinzi alone after surgery, OR
- Placebo plus platinum-containing chemotherapy prior to surgery followed by placebo alone after surgery.
At 46 months after the start of the trial:
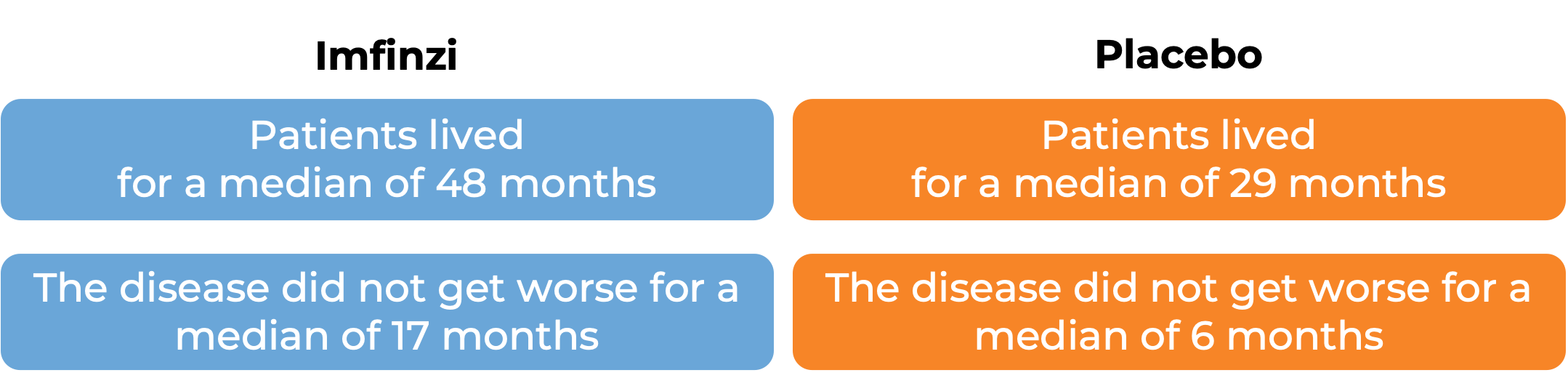
In a clinical trial, 713 patients with non-small cell lung cancer that had not spread outside the chest (Stage III), could not be removed by surgery, and who had been treated with and did not progress on at least two cycles of concurrent chemotherapy containing platinum and radiation therapy, were treated with either Imfinzi or placebo. At 33 months after the start of the trial, half of the patients treated with Imfinzi did not experience worsening of their disease for 17 months, compared to 6 months for patients treated with placebo. At 46 months after the start of the trial, 62% of patients treated with Imfinzi were alive, compared with 51% of patients treated with placebo.
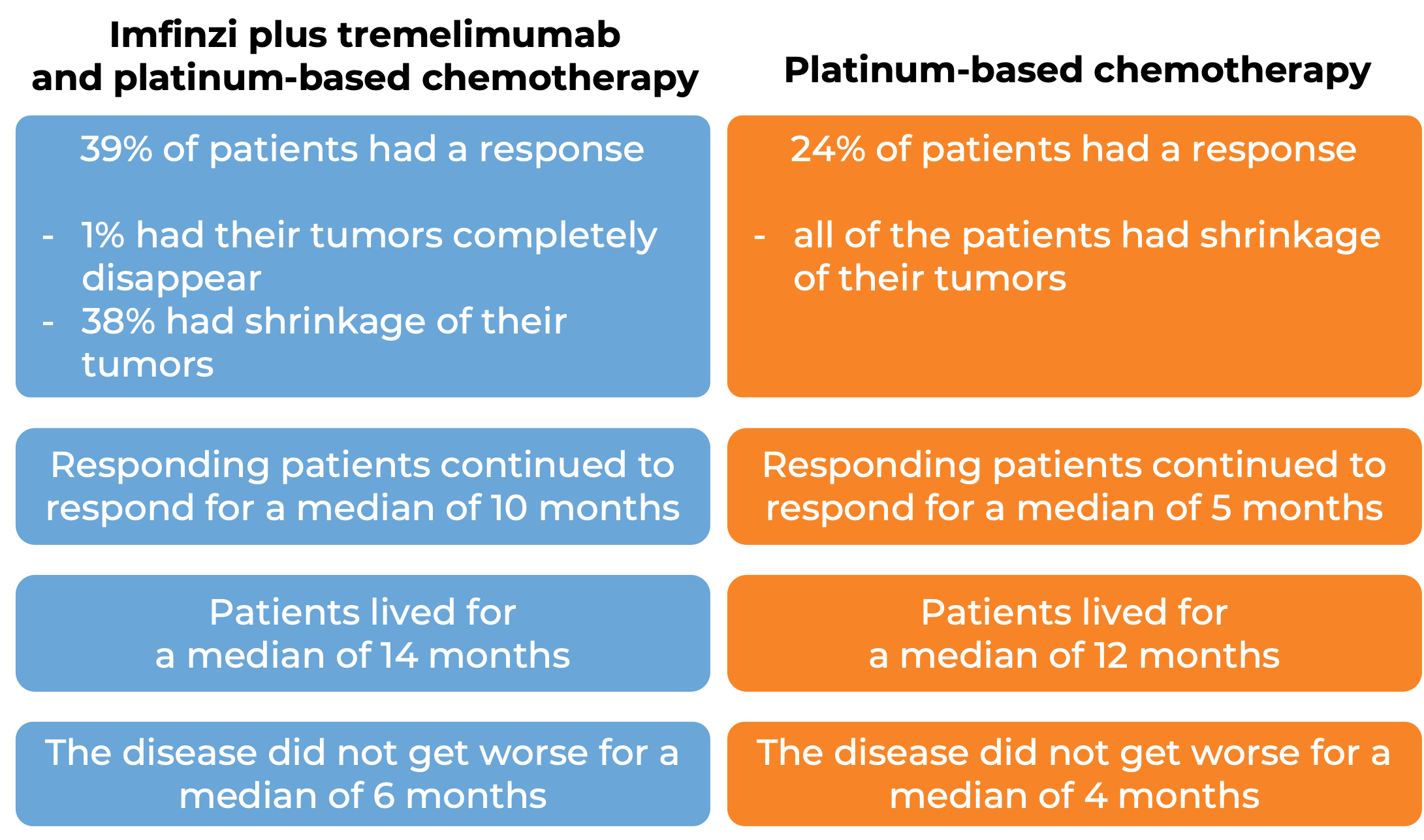
In another clinical trial, 675 patients with non-small cell lung cancer that had spread to other parts of the body and did not have an abnormal EGFR or ALK gene, were treated with Imfinzi, tremelimumab (Imjudo), and platinum-based chemotherapy, or with platinum-based chemotherapy alone.
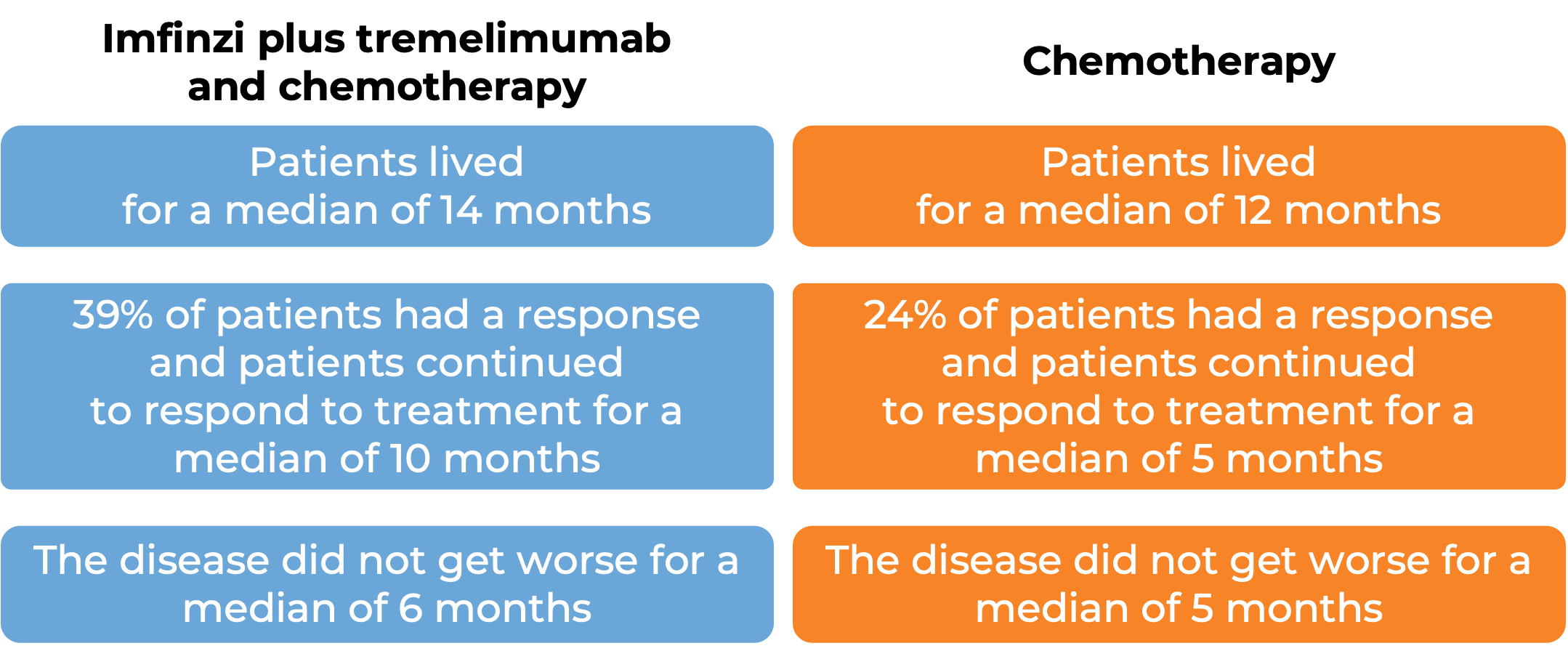
(For the definition of “median”, click here.)
Advanced small cell lung cancer
In a clinical trial, 537 patients with previously untreated extensive-stage small cell lung cancer were treated with either:
- Imfinzi, etoposide (Etopophos, Toposar), and investigator’s choice of either carboplatin (Paraplatin) or cisplatin, followed by Imfinzi alone, OR
- etoposide and investigator’s choice of either carboplatin or cisplatin
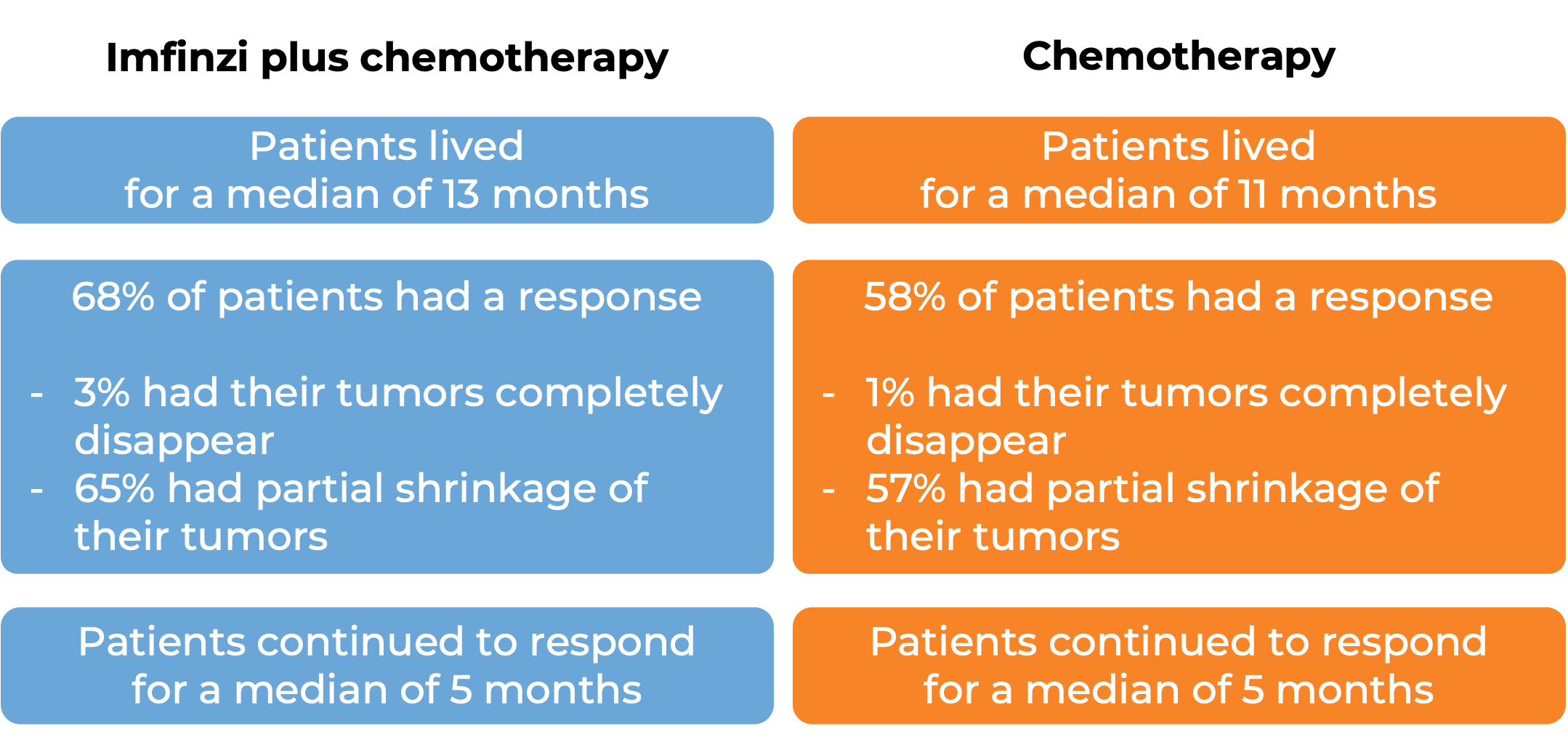
Patients treated with Imfinizi plus chemotherapy lived for a median of 13 months, compared with 10 months for patients treated with chemotherapy alone. At a median follow-up of 25 months, 68% of patients treated with Imfinzi plus chemotherapy responded to treatment, compared with 58% of patients responding to chemotherapy alone.
Biliary tract cancer
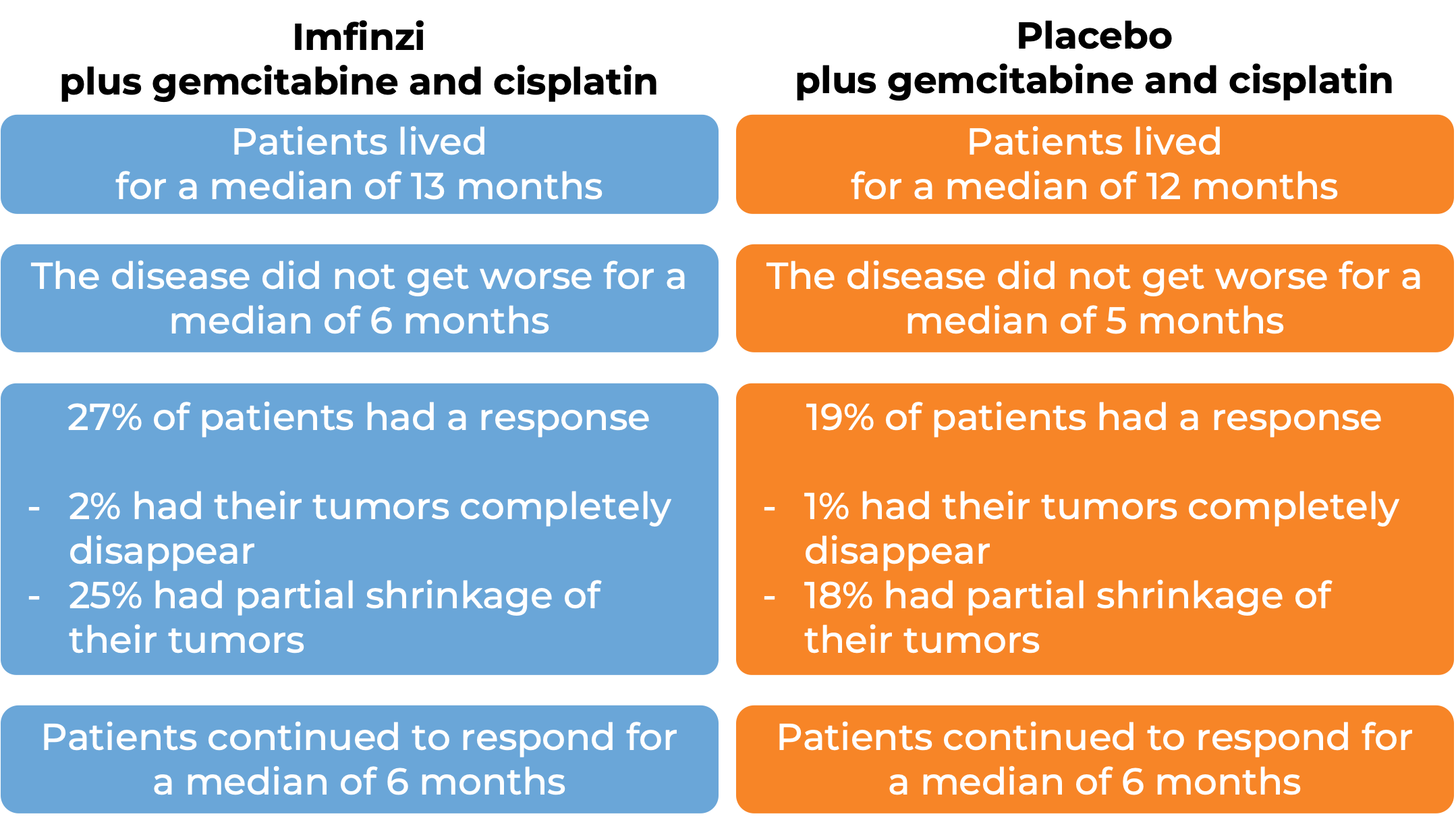
In a clinical trial, 685 patients with biliary tract cancer (including bile duct cancer/cholangiocarcinoma and gallbladder cancer) that was locally advanced and could not be removed by surgery or that had spread to other parts of the body, were treated with Imfinzi plus gemcitabine and cisplatin or with a placebo plus gemcitabine and cisplatin. At a median follow-up of 16-17 months:
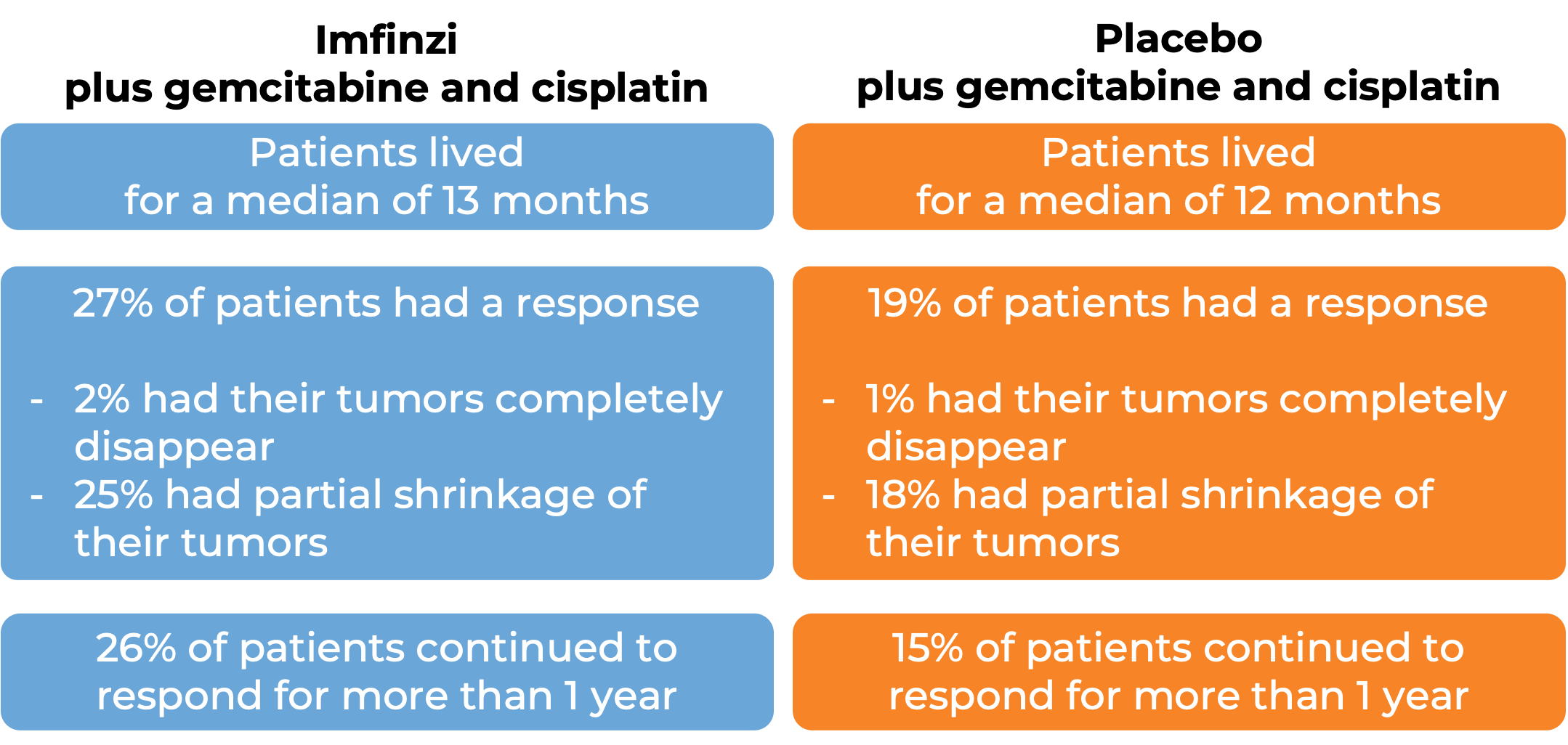
Liver cancer
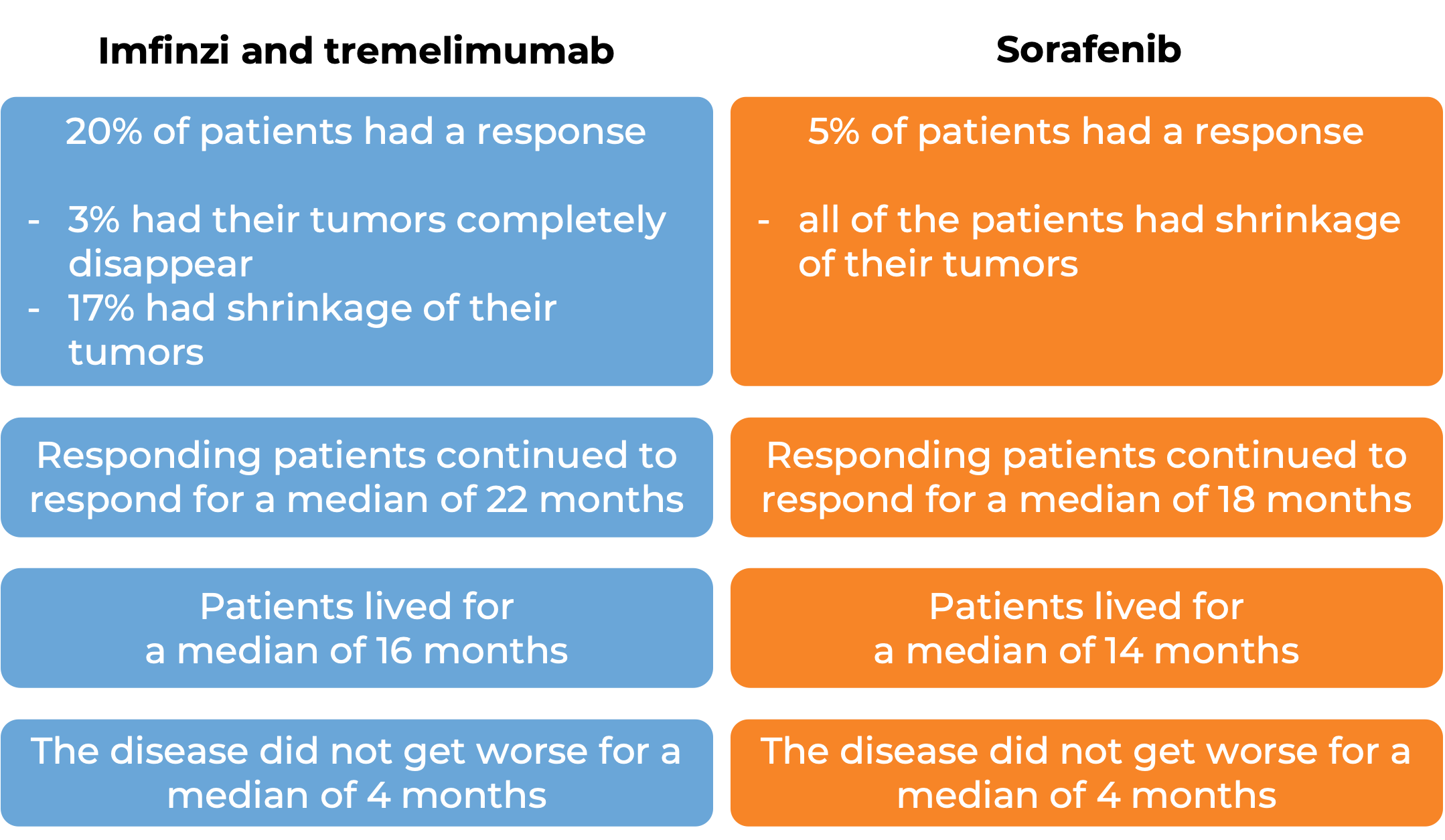
In a clinical study, 782 patients with liver cancer that could not be removed by surgery were treated with Imfinzi in combination with tremelimumab (Imjudo), or with sorafenib (Nexavar). At a median follow-up of 16 months for the Imfinzi plus tremelimumab treatment group and 13 months for the sorafenib treatment group:
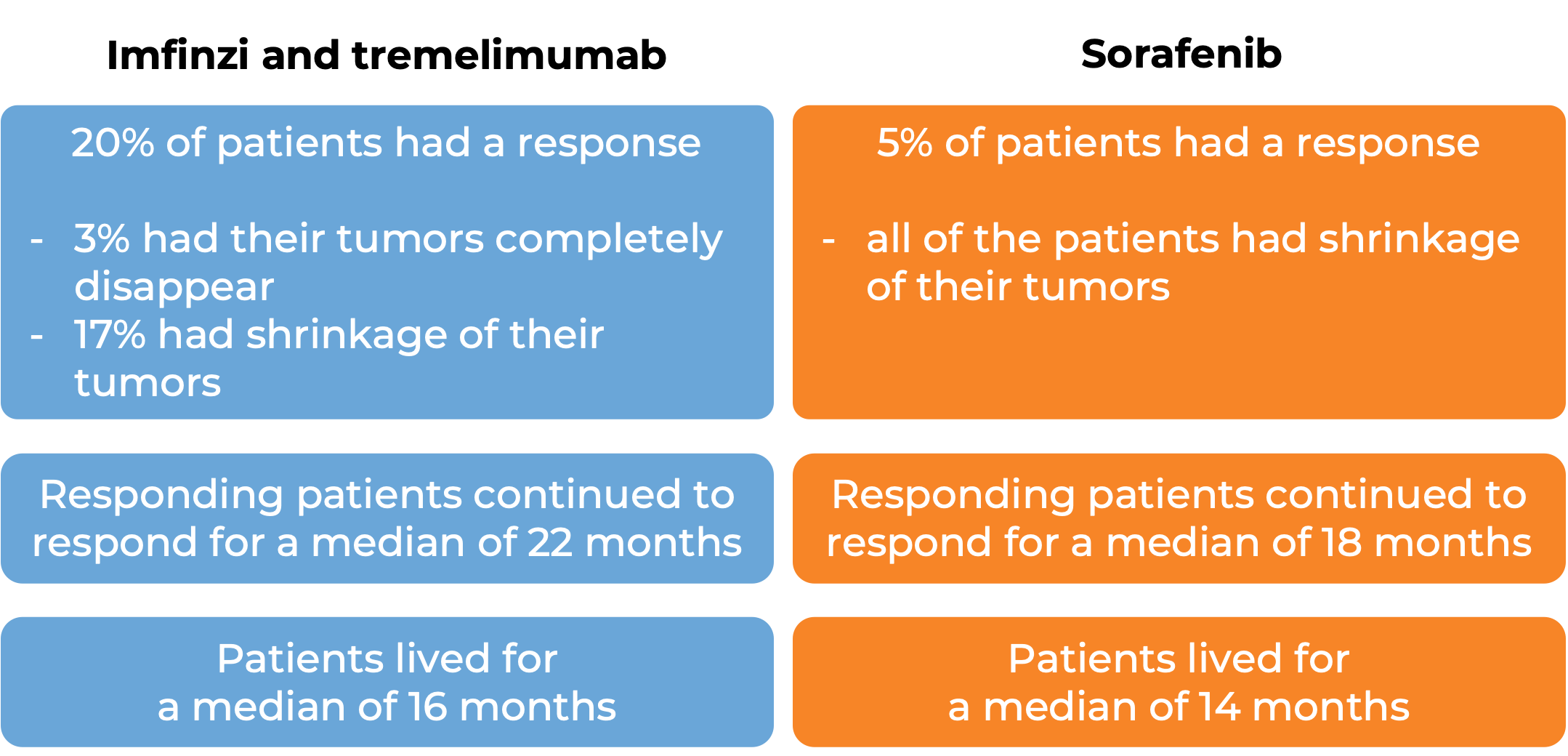
(For the definition of “median”, click here.)
Endometrial cancer
In a clinical trial, 95 patients with newly diagnosed advanced or recurrent endometrial cancer that was mismatch repair-deficient were treated with either:
- Imfinzi, at first in combination with carboplatin and paclitaxel, and then later with Imfinzi on its own, OR
- Placebo, at first in combination with carboplatin and paclitaxel, and then later with the placebo on its own.
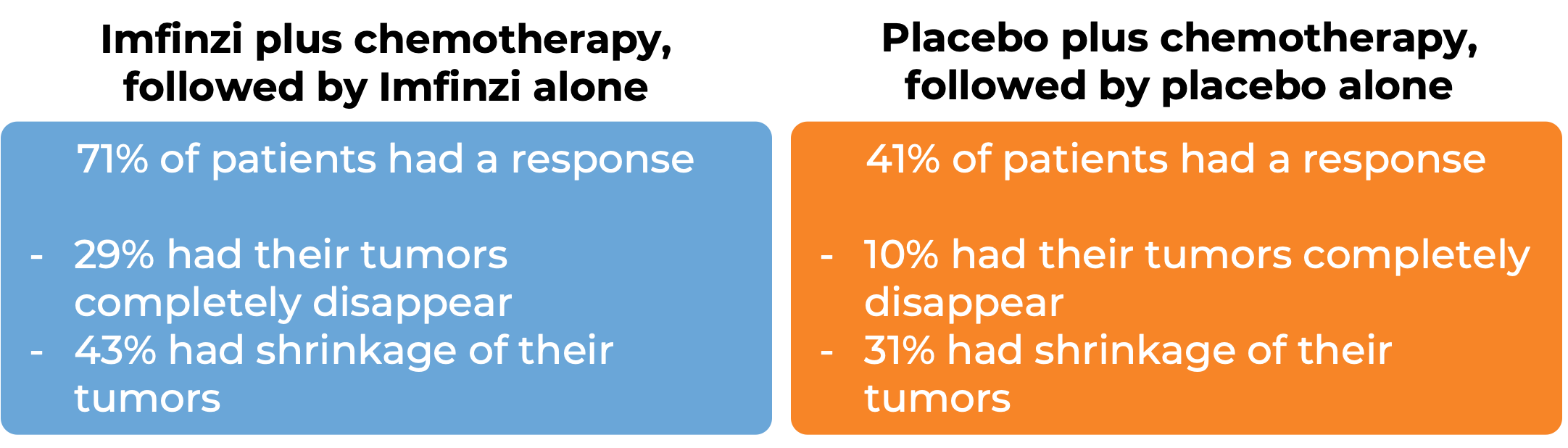
Note: Rounding percentages after the decimal can cause inconsistencies between the total and the sum of the parts.
What are the side effects?
The most common side effects of Imfinzi include fatigue, muscle and bone pain, cough, inflammation in the lungs, upper respiratory tract infections, shortness of breath, constipation, decreased appetite, nausea, swelling of the arms and legs, urinary tract infection, abdominal pain, fever, rash, and hair loss.
Imfinzi can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Imfinzi can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Imfinzi include inflammation of the lungs, liver, or colon. Additionally, problems can arise with the kidneys (including kidney failure), vision, and hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Imfinzi may cause Type 1 diabetes. Skin problems, severe infections, and reactions related to the infusion may also occur. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
AstraZeneca
Approval
FDA and EMA
Links to drug websites
Other references
- Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. Oh DY et al. NEJM Evidence (2022)
- Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. Abou-Alfa GK et al. The New England Journal of Medicine Evidence (2022)
Last updated: September 17, 2024