How is this drug name pronounced?
Dostarlimab: dos-TAR-lih-mab
Jemperli: jem-PER-lee
What cancer(s) does this drug treat?
Endometrial cancer
Jemperli is approved for:
- Patients with mismatch repair deficient (dMMR) or microsatellite instability-high (MSI-H) endometrial cancer that has spread outside the uterus (is advanced) or has returned (is recurrent). In such cases, patients are treated with Jemperli in combination with carboplatin and paclitaxel chemotherapy for six doses, followed by Jemperli alone.
- Patients with mismatch repair-deficient (dMMR) endometrial cancer who have previously been treated with a platinum-based chemotherapy and who cannot be treated by surgical removal of all known tumor sites or radiation, and whose cancer has spread outside the uterus (is advanced) or has come back (is recurrent). In such cases, Jermperli is given alone.
Mismatch repair-deficient (dMMR) cancer
Jemperli is approved for:
- Patients with advanced mismatch repair-deficient (dMMR) solid tumors who have been previously treated and the treatment either did not work or stopped working, and the cancer has gotten worse or has since come back and there are no other treatment options.
Limitations of use:
Age: The safety and efficacy of Jemperli in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Jemperli can cause harm to a fetus and is not recommended for use during pregnancy. Pregnancy should be prevented during treatment with Jemperli and for at least four months after the last dose of Jempeli. The risks associated with Jemperli during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for at least four months after the last dose of Jemperli.
Complications of stem cell transplant: Serious and life-threatening transplant-related complications can occur in patients who receive a stem cell transplant from a donor before or after being treated with Jemperli.
What type of immunotherapy is this?
- PD-1 blockade
How does this drug work?
- Target: PD-1
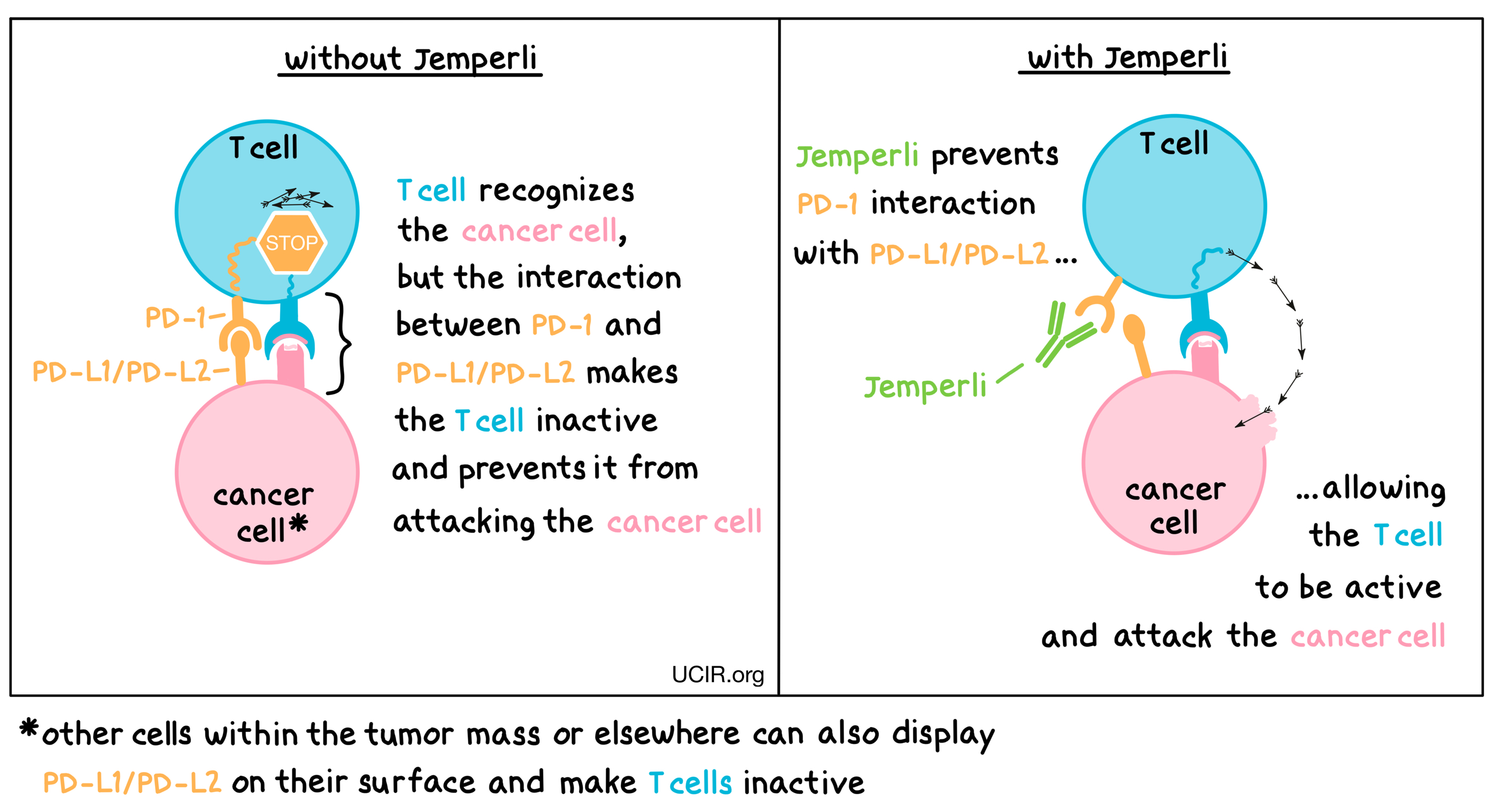
Jemperli is an antibody that attaches to a molecule called PD-1, which is present on the surface of T cells. In healthy T cells, PD-1 acts as a brake that prevents the cells from creating an out-of-control immune response. However, in tumors, PD-1 can make T cells inactive and can prevent them from doing their job of killing the cancer cells. This happens because cancer cells or other cells within the tumor have an increased concentration of PD-L1 and PD-L2 molecules on their cell surface, which attach to PD-1. When PD-L1 and PD-L2 interact with the PD-1 receptor on the T cell, the T cell is inactivated and cannot do its job of killing the cancer cells. Jemperli binds to the PD-1 on the T cells in a way that blocks PD-L1 and PD-L2 from interacting with the PD-1 receptor. This blockade on PD-1 allows the T cells to activate and to attack and kill cancer cells.
How is this drug given to patients?
Jemperli is administered via a tube into a vein (intravenous infusion or i.v.) over the course of 30 minutes. When Jemperli is being used alone, it is administered every three weeks for the first four doses, then every 6 weeks after that. When used in combination with carboplatin and paclitaxel, Jemperli is administered every three weeks followed by treatment with carboplatin and paclitaxel on the same day, for the first six doses. After that, Jemperli is given alone every 6 weeks.
What are the observed clinical results?
For:
Endometrial cancer (untreated or previously treated)
Endometrial cancer (previously treated with a platinum-based chemotherapy)
Mismatch repair-deficient (dMMR) cancer
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Endometrial cancer (previously treated with a platinum-based chemotherapy):
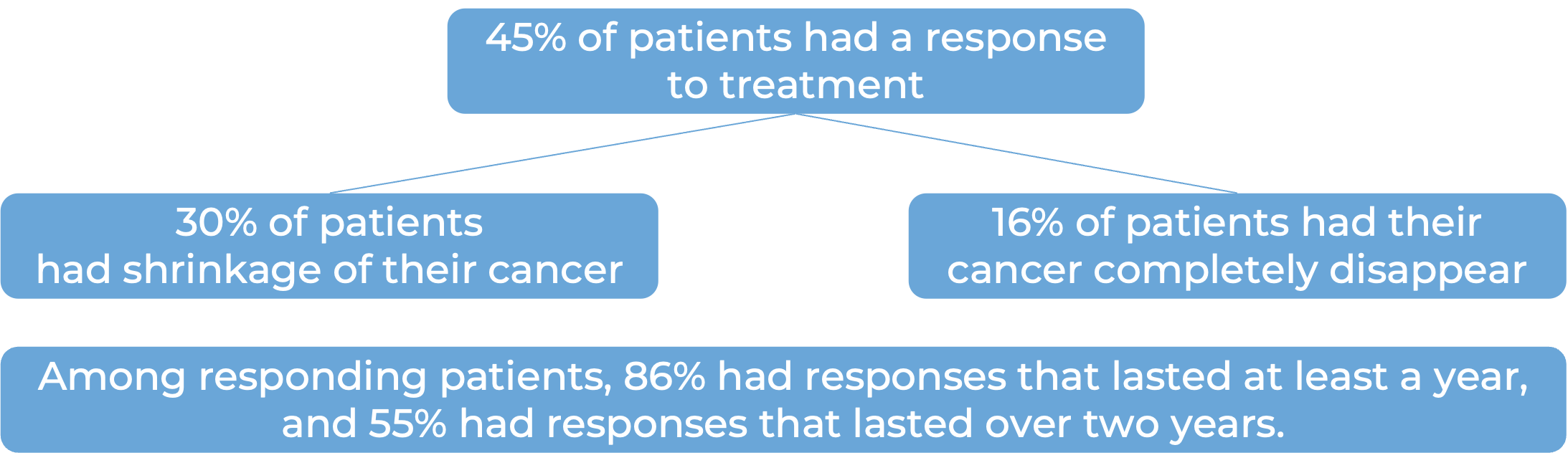
In a clinical trial, 141 patients with mismatch repair- deficient (dMMR) endometrial cancer that was recurrent or advanced, and had previously been treated with a platinum-based chemotherapy, were treated with Jemperli.
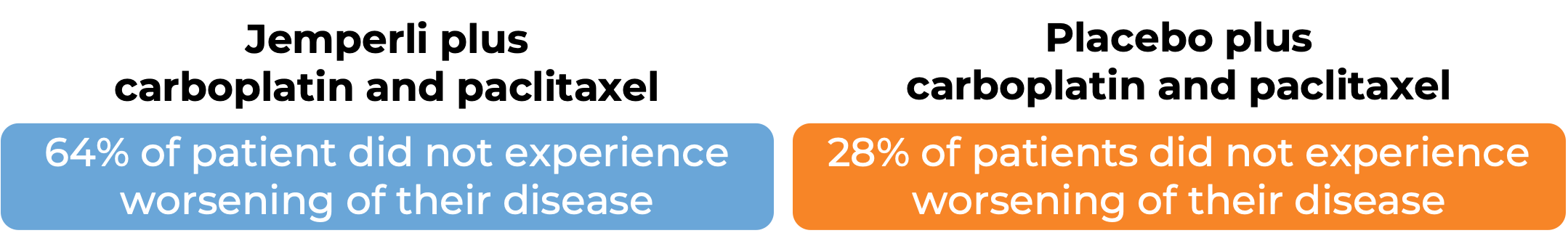
Endometrial cancer (untreated or previously treated):
In a clinical trial, 122 patients with advanced or recurrent mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) endometrial cancer that could not be treated with curative surgery or radiation, were treated with either:
- Jemperli plus carboplatin and paclitaxel for six doses, followed by Jemperli alone, OR
- placebo plus carboplatin and paclitaxel for six doses, followed by placebo alone.
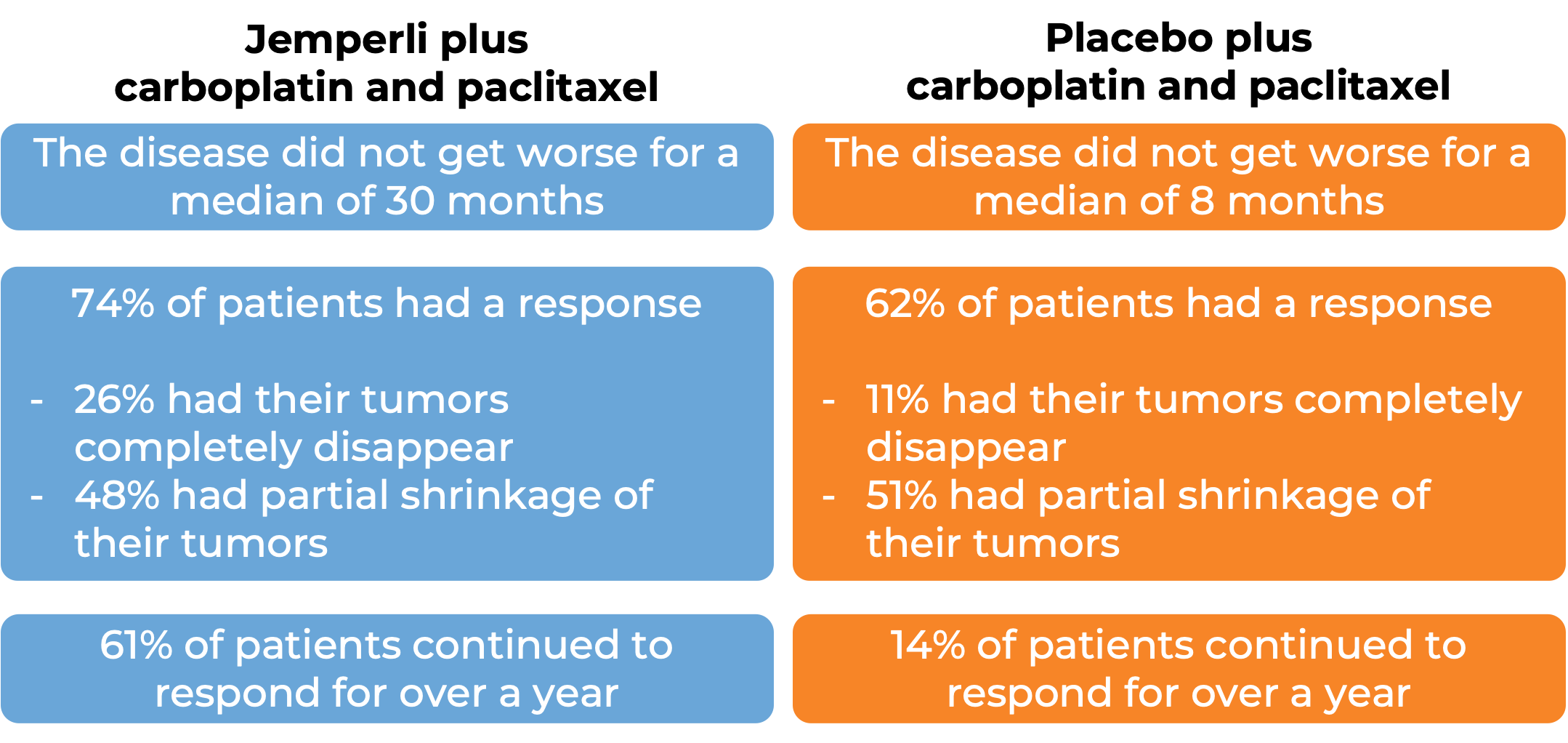
Mismatch repair-deficient (dMMR) cancer
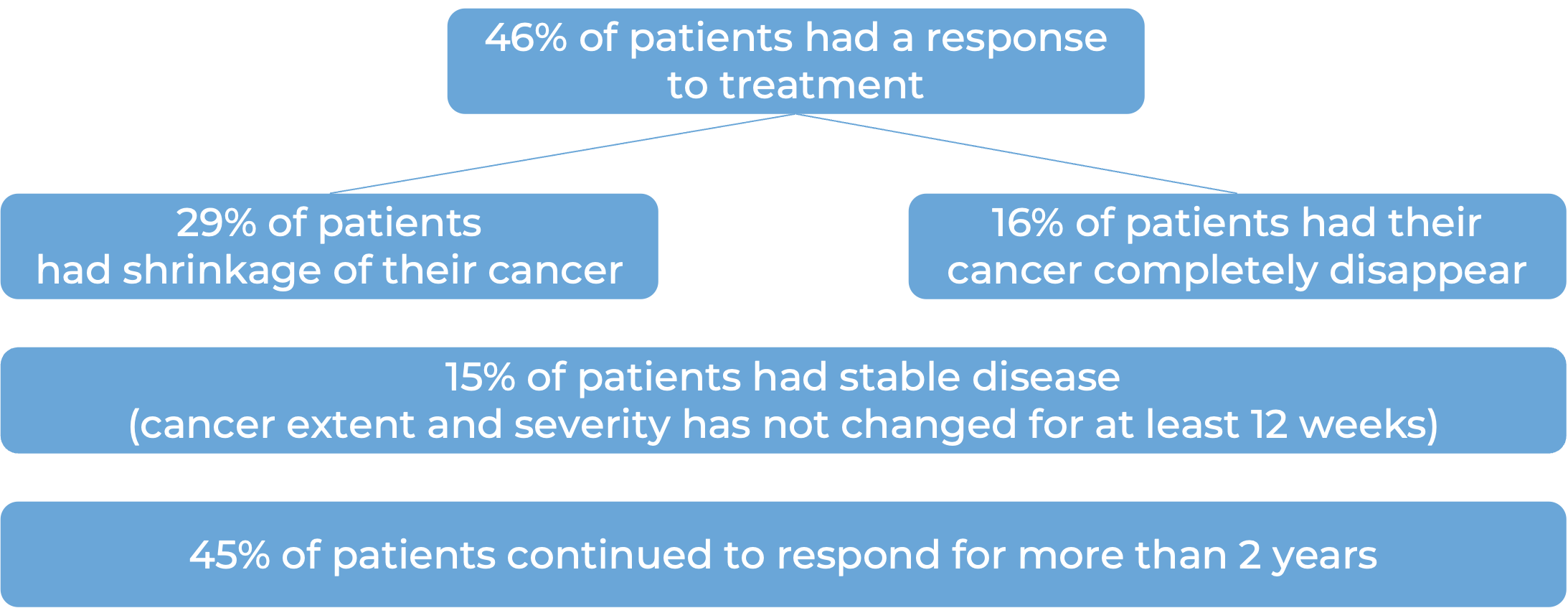
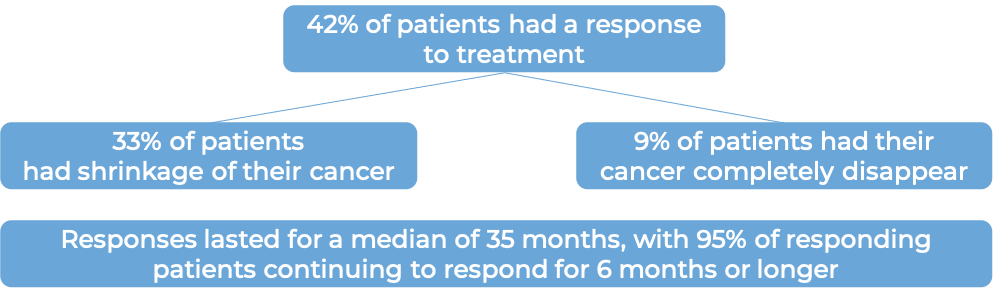
In a clinical trial, 209 patients with advanced, previously treated, mismatch repair-deficient (dMMR) solid tumors (including colorectal cancer, small intestinal cancer, gastric cancer, pancreatic cancer, biliary neoplasm, liver cancer, ovarian cancer, adrenal cortical cancer, breast cancer, esophageal cancer, genital neoplasm, renal cell carcinoma, and others) who had no other treatment options, were treated with Jemperli.
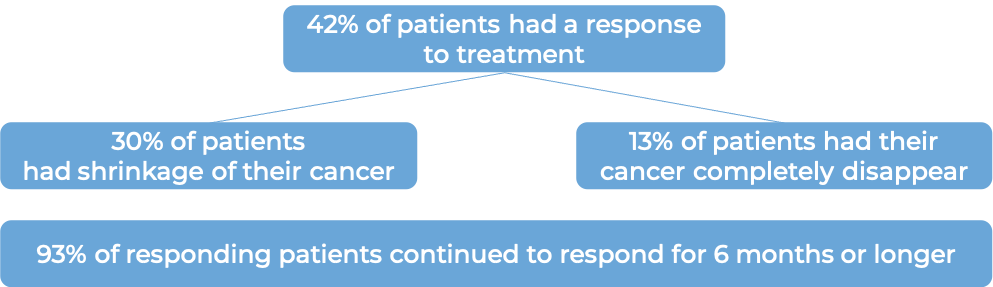
In another clinical trial, 71 patients with advanced mismatch repair-deficient (dMMR) endometrial cancer who had been previously treated with platinum-containing chemotherapy, and the treatment either did not work or stopped working and the cancer had gotten worse or had since come back, were treated with Jemperli. At a median follow-up of 14 months:
What are the side effects?
The most common side effects of Jemperli alone are fatigue, nausea, diarrhea, and low red blood cell counts (anemia). The most common side effects for Jemperli used in combination with carboplatin and paclitaxel are rash, diarrhea, hypothyroidism (underactive thyroid gland), and high blood pressure.
Jemperli can cause a patient’s T cells to attack healthy cells throughout the body. Because of this, Jemperli can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Jemperli include inflammation of the lungs, colon, liver, or kidneys. Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Jemperli may cause Type 1 diabetes. Serious skin problems, severe infections, and reactions related to the infusion may also occur.
Jemperli can cause serious and life-threatening complications, including graft-versus-host disease in patients who have received a stem cell transplant before or after being treated with Jemperli. These complications may arise even if patients have been treated with other types of therapy in between administration of Jemperli and stem cell transplant.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer:
- GlaxoSmithKline
Approval:
- FDA and EMA
Links to drug websites:
- US: https://www.jemperlihcp.com/
- Europe: https://www.ema.europa.eu/en/documents/product-information/jemperli-epar-product-information_en.pdf
Last updated on August 16, 2023